Introduction
Eales’ disease is an idiopathic retinal periphlebitis that primarily affects the peripheral retina in young adults. Eales’ disease was first described by Henry Eales, a British ophthalmologist, in 1880 and 1882.1,2 He found it in seven young, male patients ranging in age from 14 to 29 years with recurrent vitreous hemorrhage. In addition, these patients had history of headache, variation in peripheral circulation, chronic constipation and epistaxis. In the next century, the disease was redefined by several investigators. 3-6 Elliot first recognized the inflammation of retinal vein and described it as periphlebitis retinae.4 Subsequently several investigators documented both venular and arteriolar inflammation.5,7
Eales’ disease most commonly affects healthy young adult males and is an important cause of preventable blindness in young adults. The predominant age of onset of symptoms is 20-30 years. The disease is more commonly seen in the Indian subcontinent. However, it has been reported from United Kingdom, U.S.A., Canada, Germany, Greece and Turkey.
Eales’ disease is characterized by retinal periphlebitis (Fig. 1), peripheral retinal ischemia (Fig. 2), and neovascularization (Fig.3). Visual loss is characteristically caused by recurrent vitreous haemorrhage.8 Vascular involvement in Eales’ disease may be peripheral or central. Central Eales’ disease is markedly uncommon (Fig. 4). 9-11
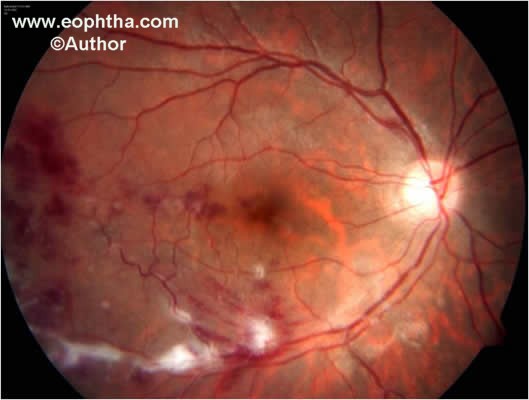
Fig. 1.Color fundus photograph shows retinal periphelbitis along with superficial retinal hemorrhages.
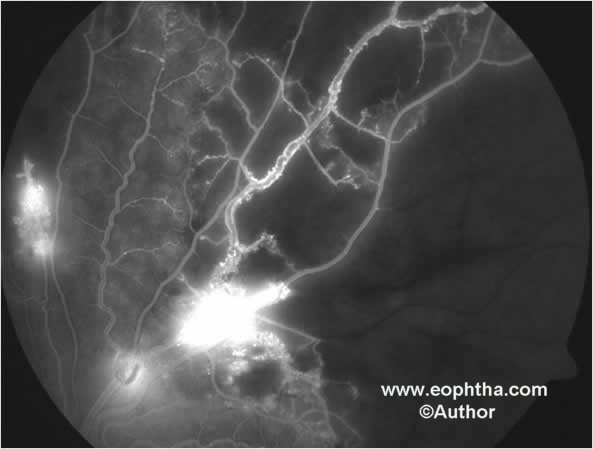
Fig. 2.Fundus fluorescein angiography shows capillary non perfusion, microaneurysms and leakage of fluorescein dye from retinal neovascularization.
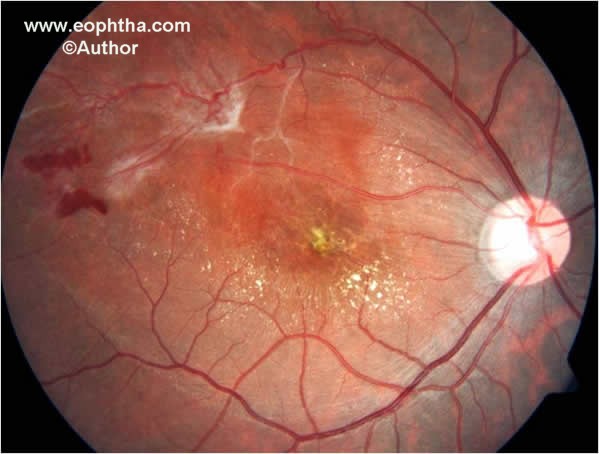
Fig. 3.Color fundus photographs shows neovascularization elsewhere, sheathing of retinal vessels and hard exudates at macula.
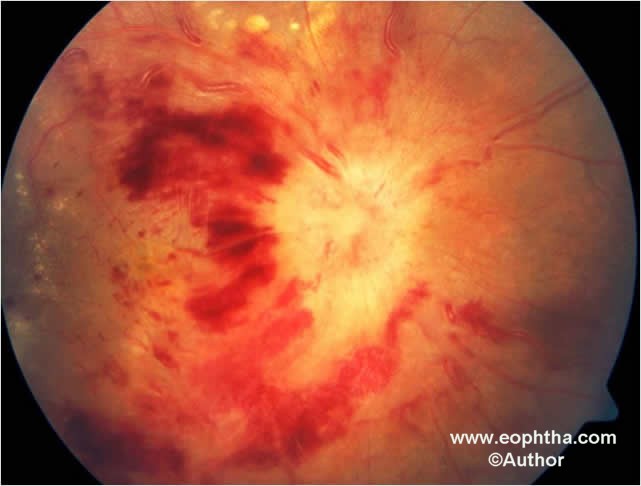
Fig. 4.Color fundus photographs shows Central Eales’ disease.
Etiopathogenesis
Eales’ disease appears to be an immunologic reaction that may be triggered by an exogenous exposure. Retinal S-antigen and Interphotoreceptor Retinoid Binding Protein play a role in the etiopathogenesis of this condition. An extraneous agent results in the exposure of normally sequestered uveitopathogenic antigens of the immune system, leading to an immune response in the eye that initiates the disease process. 12 Oxidative stress has been found to play an important role in the etiopathogenesis. 13-20 Lowered levels of antioxidant vitamins E and C and consequent accumulation of oxygen and lipid free radicals, or vice versa, could explain the inflammation, neovascularization and retinal pathology in patients with Eales' disease. Also, vitamin A deficiency could aggravate retinal illness. Elevated lipid peroxides have been found in the proliferative stage, which induce synthesis of cytokines and growth factors in retina during neovascularization.15
Eales’ disease is distinctively characterized both by stage of inflammation as well as stage of proliferation.7 Cytokines play an important role in intraocular inflammation.8,9 The cascade of multiple angiogenic cytokines induced by oxidative damage, associated with tissue hypoxia, may interact to promote sustained retinal neovascularization.10 During the inflammatory and proliferative stages of the disease statistically significant increase in IL-1b, IL-6, IL-10 and TNF-a expression was observed as compared to controls, highlighting the role of pro- and anti-inflammatory cytokines in the pathogenesis.21
Markedly raised levels of IL-1b and TNF-a have been observed in the inflammatory stage which persisted in the proliferative stage. Raised levels of IL-1b, in the inflammatory stage, decreased significantly in the proliferative stage. Elevated TNF-a levels, observed in the inflammatory stage, increased significantly in the proliferative stage where clinically, inflammation (periphlebitis) had subsided but retinal neovascularization and vitreous hemorrhage had developed with the occurrence of retina hypoxia and ischemia. This data suggests that despite clinical absence of periphlebitis in the proliferative stage of the disease, IL-1b and TNF-a levels remain raised significantly as compared to controls. The synergism of IL-1 and TNF is a commonly reported phenomenon. IL-1 and TNF initiate the cascade of inflammatory mediators by targeting the endothelium. Although the receptors for TNF and IL-1 are clearly different, the post receptor events are similar. IL-1 often synergizes with TNF for NO induction which mediates cell death.11 Nitrosoactive stress has been found to promote retinal vasculitis in Eales’ disease.12 Significant TNF-a expression was observed during the proliferative stage. These findings indicate that angiogenesis during the proliferative stage is induced by TNF-a. Angiogenesis induced by TNF-a, during post-ischemic inflammation, may be modulated through induction of potent angiogenic factors.14 Hypoxia-induced expression of vascular endothelial growth factor is only one aspect of the complicated processes in intraocular neovascularization.15 Chemokines have also been found to be involved in the recruitment of neutrophils and monocytes into the vitreous and play a role in the intraocular neovascularization.16 Thus, the IL-1 system represents a novel target for controlling inflammatory activity and/or the associated long-term sequelae related to angiogenesis in Eales’ disease. Role of TNF-a in the inflammatory as well as proliferative stage of the disease has implications for anti-TNF-a therapy in Eales’ disease. Reducing the biological activities of IL-1 and TNF may be accomplished by several different, but highly specific strategies, which involve neutralizing antibodies, soluble receptors, receptor antagonist, and inhibitors of proteases that convert inactive precursors to active, mature molecules. Anti-cytokine therapeutic agents such as TNF-neutralizing antibodies, soluble TNF receptors, and IL-1 receptor antagonist may prove beneficial. Infliximab (anti-TNF-a) may prove to be beneficial in patients of Eales’ disease.21
A close relationship between the prominent neovascular proliferation in Eales' disease and the intense expression of VEGF has been found. The increased expression of VEGF, when compared to other conditions inducing neovascularization, might explain the severity of neovascular growth and the propensity of repeated vitreous hemorrhage in Eales' disease.22
Retinal photoreceptors and platelets have been shown to be an easy target of oxidants because of high proportion of polyunsaturated fatty acids. The decreased membrane fluidity in platelets suggests alterations in the physiological events, which may result in alterations in functioning of retinal photoreceptors.20
Mycobacterium tuberculosis DNA has also been detected by polymerase chain reaction, in the vitreous of such patients. 23-25 However, the role of mycobacterium genome in the pathogenesis is yet to be ascertained.
Clinical Features
Eales’ disease with a characteristic clinical picture, fluorescein angiographic findings and natural course is considered a specific disease entity. Recurrent vitreous hemorrhage is the hall mark of this disease. A new classification system has been proposed recently by the author.26 This staging system, based on standard terminology and features, provides a simple method to categorize, according to the severity of the disease. This staging system takes into consideration the fundoscopic and fluorescein angiographic variables that have been shown to be prognostic of visual outcome (Table). Macular involvement is uncommon. Macular ischemia and traction macular detachment are associated with poor visual outcome (Fig. 5).27
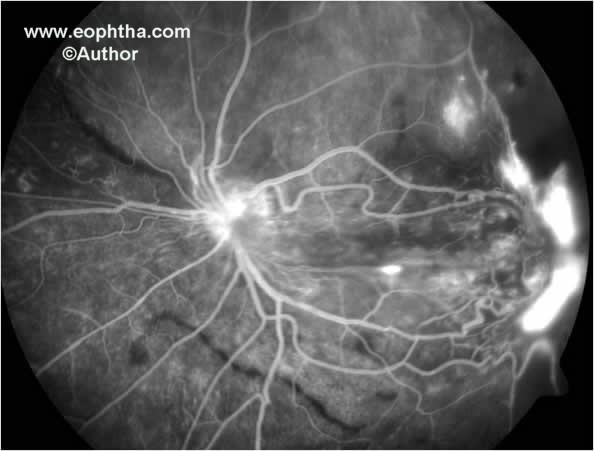
Fig. 5.Fundus fluorescein angiography shows traction macular detachment, neovascularization elsewhere associated with capillary non-perfusion and early neovascularization at the disc.
Table. The classification system for Eales’ disease.
___________________________________________________________
Stages Features ___________________________________________________________
A. Eales’ disease
Stage 1a Periphlebitis of small caliber vessels with
superficial retinal hemorrhages
Stage 1b Periphlebitis of large caliber vessels with
superficial retinal hemorrhages
Stage 2a Peripheral capillary nonperfusion
Stage 2b Neovascularization elsewhere / Neovascularization
of the disc
Stage 3a Fibrovascular proliferation
Stage 3b Vitreous hemorrhage
Stage 4a Traction / combined rhegmatogenous detachment
Stage 4b Rubeosis iridis, neovascular glaucoma, complicated
cataract, and optic atrophy
B. Central Eales’ disease
________________________________________________________________________
The new classification system is consistent, simple and easy to recall. It can also be used to monitor the effect of medical, laser and/or surgical treatment.
Fluorescein Angiography
In cases of active retinal periphlebitis, staining of the veins can be seen in the early venous phase with extravasation of dye in the late phase (Fig. 6). In the healed stage, only staining of the vessel wall occurs without any leak in the late venous phase. Areas of capillary non-perfusion and retinal neovascularization can be easily delineated by fluorescein angiography. Capillary non-perfusion of more than 20 disc area and 60 disc area are associated with neovascularization elsewhere (NVE) and neovascularization of the disc (NVD). 28 Retinal neovascularization sites have been found to cluster around specific anatomic foci. The quadrantic distribution of the sites of NVE in Eales’ disease is: superotemporal, 45.95%; inferotemporal, 24.32%; superonasal, 16.22%; and inferonasal, 13.51%.29
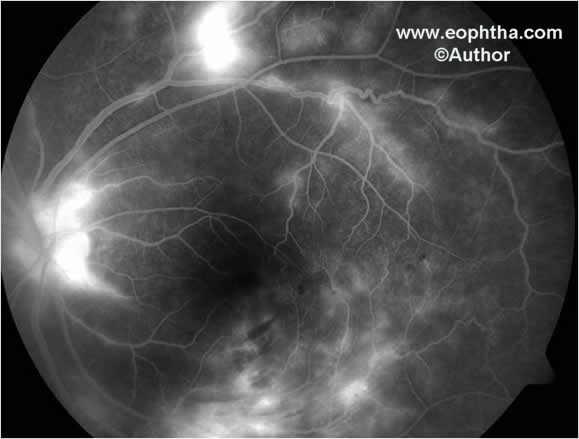
Fig. 6. Fundus fluorescein angiography shows leakage of fluorescein dye from neovasculazation at the disc, neovascularization elsewhere and areas of retinal periphlebitis.
Central Eales’ disease is relatively uncommon. Such central retinal periphlebitis has a similar presentation as central retinal vein occlusion. Branch retinal vein occlusion with peripheral retinal periphlebitis (Fig. 7) and central Eales’ disease have been found to be associated with favorable visual outcome.30,31
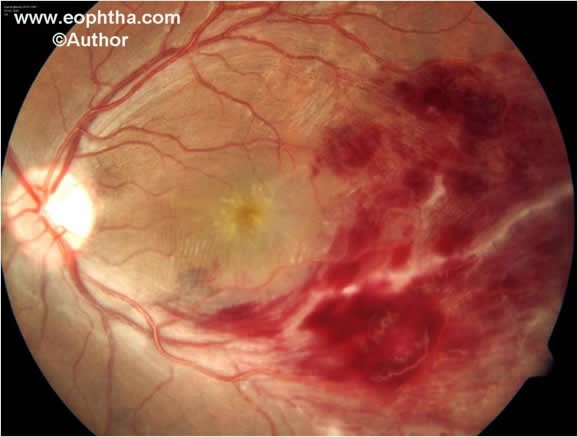
Fig. 7. Color fundus photograph shows inferotemporal retinal vein occlusion, retinal periphlebitis and macular edema.
Management
The management of Eales’ disease depends on the severity of the disease. Management strategies can also be defined according to the stage of the disease. Stage 1, the stage of inflammation, is amenable to medical therapy. Stage 2, the stage of ischemia and neovascularization, requires observation/laser photocoagulation. Stage 3, the stage of proliferation, requires laser / pars plana vitrectomy and laser. Stage 4, the stage of complications, requires sophisticated surgical management strategies.26
a. Observation
Patients with inactive retinal periphlebitis can be observed periodically at 6-month interval. Patients with fresh vitreous hemorrhage can also be observed at intervals of 4-6 weeks if underlying retina is found to be attached by indirect ophthalmoscopy or by ultrasound.
b. Medical Therapy
Corticosteroids:Anti-inflammatory corticosteroid drugs are potent therapeutic agents for a wide range of ocular and systemic disorders and remain the mainstay of therapy in retinal periphlebitis in Eales’ disease (1mg/kg/day).
Methotrexate:Predominantly T-cell involvement has been demonstrated in the lymphocytic infiltration of epiretinal and subretinal membranes in Eales’ disease. Hence, treatment should be directed to the down regulation of the activated T-cells. Search for safer and more specific forms of treatment have lead to certain immunosuppressives, like methotrexate, which have found a role in patients with immunologically driven systemic diseases. As opposed to the more ‘cytostatic’ effects of corticosteroids, the ‘cytotoxic’ immunosuppressives exert their beneficial effects by actually killing the rapidly dividing clones of lymphocytes that are responsible for inflammation. Methotrexate, a folic acid antagonist, has anti-inflammatory and immunomodulatory actions. The drug reduces the synthesis of DNA by acting on the enzyme dihydrofolate reductase. Methotrexate is used as a weekly 'pulsed' therapy. A 'pulse' differs from chronic moderate dose therapy in its ability to "reset" an aberrant immune response. Inhibition of the proliferating lymphocyte clones, the temporary removal of recirculating T-lymphocytes from the blood and eye, and the profound suppression of peripheral inflammation, all occur simultaneously. Antigens exposed by viral, bacterial or autoimmune injury are normally perpetuated by the inflammatory response but in such a system a pulse may abolish the source of antigen at the same time as it suppresses the immune response. When memory T-cells recirculate, the disease falters in the absence of the antigen. 32,33
c. Photocoagulation
Photocoagulation is the mainstay of therapy in proliferative stage of the disease.34 Laser photocoagulation leads to resolution of retinal neovascularization due to its anti-VEGF effect. In cases of gross capillary non-perfusion photocoagulation is suggested. For NVE and NVD, sectoral scatter photocoagulation and pan retinal photocoagulation, respectively is suggested (Fig. 8).
d. Vitreoretinal Surgery
Vitrectomy alone or combined with other vitreoretinal surgical procedures is often required. Vitrectomy for non-resolving vitreous hemorrhage done at 3-6 months has better visual outcome than done after 6 months. Patients with fewer episodes of vitreous hemorrhage and preoperative laser have better visual prognosis.35,36
e. Anterior Retinal Cryo Ablation
Anterior retinal cryo ablation is usually reserved as an adjunct to photocoagulation in Eales’ disease for effect in peripheral retina.
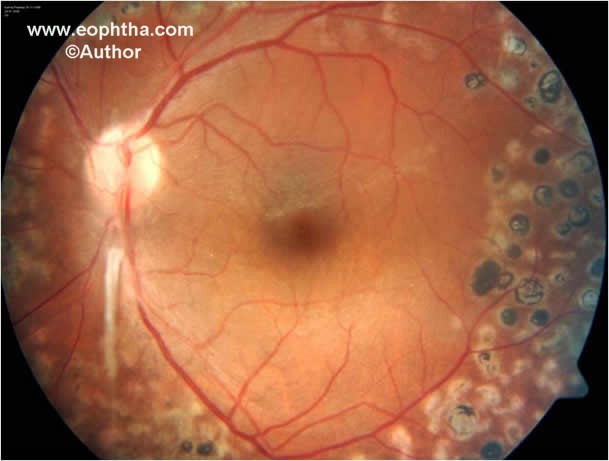
Fig. 8. Color fundus photograph after laser photocoagulation shows residual fibrous proliferation from the disc following resolution of neovascularization at the disc.
References
1. Eales H. Retinal haemorrhage associated with epistaxis and constipation. Brim Med Rev 1880;9:262.
2. Eales H. Primary retinal haemorrhage in young men. Ophthalmic Rev 1882;1:41.
3. Duke-Elder S, Dobree JH. Primary perivasculitis of the retina: Eales disease, in Duke Elder, Perkins EJ (eds): System of Ophthalmology, Vol. X. London, Henry Kimpton, 1967, pp 222-236.
4. Elliot AJ. 30-year observation of patients with Eales disease. Am J Ophthalmol 1975;80:404-408.
5. Kimura SJ, Carriker FR, Hogen MJ. Retinal vasculitis with intraocular hemorrhage. Classification and results of special studies. Arch Ophthalmol 1956;56:361-365.
6. Cross AG. Vasculitis retinae. Trans Ophthalmol Soc UK 1963;83:133.
7. Keith-Lyle T, Wybark A. Retinal vasculitis. Br J Ophthalmol 1961;45:77-78.
9. Gilbert TW. Periphlebitis and endovasculitis of retinal vessels. Klin Monatsbl Augenheilkd 1935;94:335-349.
9. Kumar D, Saxena RC, Saxena S. Vitreous hemorrhage in Eales’ disease. Afro-Asian J Ophthalmol 1995;13:109-112.
10. Atmaca LS, Idil A, Gunduz K. Visualization of retinal vasculitis in Eales disease. Ocul Immunol Inflammol 1993;1:41-48.
11. Das T, Biswas J, Kumar A, Namperumalsamy P, Nagpal PN, Patnaik B, Tewari HK. Eales’disease. Ind J Ophthalmol 1994;42:3-18.
12. Saxena S, Rajasingh J, Biswas S, Kumar D, Shinohara T, Singh V.K. Cellular response to Retinal S-antigen and Interphotoreceptor Retinoid Binding Protein fragments in patients with Eales' disease. Pathobiology 1999;67:37-44.
13. Saxena S, Khanna VK, Kumar D, Srivastava P, Seth PK. Enhanced oxidative stress in Eales’ disease. Ann Ophthalmol 2001;33:40-42.
14. Saxena S, Kumar D, Srivastava P, Khanna VK, Seth PK. Low levels of platelet glutathione in Eales' disease. Med Sci Res 1999;42:125-126.
15. Srivastava P, Saxena S, Khanna VK, Kumar D, Nath R, Seth PK. Raised platelet thiobarbituric acid reacting substances in proliferative Eales disease. Indian J Ophthalmol 2000;48:307-310.
16. Saxena S, Khanna VK, Kumar D, Srivastava P, Seth PK. Impaired antioxidant defense mechanism in central Eales’ disease. Ann Ophthalmol 2004;36:29-31.
17. Bhooma V, Sulochana KN, Biswas J, Ramakrishnan S. Eales’ disease: accumulation of reactive oxygen intermediates and lipid peroxides and decrease of antioxidants causing inflammation, neovascularization and retinal damage. Curr Eye Res 1997;16:91-95.
18. Sulochana KN, Biswas J, Ramakrishnan S. Eales’ disease: increased oxidation and peroxidation products of membrane constituents chiefly lipids and antioxidant enzymes and reduced glutathione in vitreous. Curr Eye Res 1999;19:254-259.
19. Swamy-Mruthinti S, Miriam KC, Kumar SK, Biswas J, Ramakrishnan S, Nagaraj RH, Sulochana KN. Immunolocalization and quantification of advanced glycation end products in retinal neovascular membranes and serum: a possible role in ocular
neovascularization. Curr Eye Res 2002;25:139-145.
20. Saxena S, Srivastava P, Kumar D, Khanna VK, Seth PK. Decreased platelet membrane fluidity in retinal periphlebitis in Eales’ disease. Ocul Immunol Inflamm 2006;14:113-116.
21. Saxena S, Pant AB, Khanna VK, Agrawal AK, Singh K, Kumar D, Singh VK. Interleukin-1 and Tumor necrosis factor- alpha: novel targets for immunotherapy in Eales’ disease. Ocular Immunology Inflammation 2009;17:201-206.
22. Perentes Y, Chan CC, Bovey E, Uffer S, Herbort CP. Massive vascular endothelium growth factor (VEGF) expression in Eales' disease. Klin Monatsbl Augenheilkd. 2002;219:311-314.
23. Madhavan HN, Therese KL, Doraiswamy K. Further investigations on the association of Mycobacterium tuberculosis with Eales’ disease. Ind J Ophthalmol 2002;50:35-39.
24. Biswas J, Therese L, Madhavan HN. Use of polymerase chain reaction in detection of Mycobacterium tuberculosis complex DNA from vitreous samples of Eales’ disease. Br J Ophthalmol 1999;83:994.
25. Madhavan HN, Therese KL, Gunisha P, Jayanti U, Biswas J. Polymerase chain reaction for detection of Mycobacterium tuberculosis in epiretinal membrane in Eales’ disease. Invest Ophthalmol Vis Sci 2000;41:822-825.
26.Saxena S, Kumar D. A new staging system of idiopathic retinal periphlebitis. Eur J Ophthalmol 2004;14:236-239.
27. Saxena S, Kumar D. Macular involvement in Eales’ disease. Ann Ophthalmol 2000;32:98- 100.
28. Saxena S, Kumar D, Maitreya A, Srivastava P, Khanna VK, Nath R, Seth PK. Ann 2005;37:273-275.
30. Saxena S, Kumar D. Visual outcome in Eales’ Disease with Branch Retinal Vein Occlusion. Ann Ophthalmol 1999;31:173-175.
31. Saxena S, Kumar D. Visual outcome in Central Eales’ disease. Ann Ophthalmol 2001;33:300-302.
32. Saxena S, Kumar D, Kapoor S. The efficacy of oral methotrexate pulsed therapy in Eales’ disease. Ann Ophthalmol 2000;32:60-62.
33. Bali T, Saxena S, Kumar D, Nath R. Response time and efficacy of oral methotrexate pulsed therapy in idiopathic retinal periphlebitis. Eur J Ophthalmol 2005;15:374-378.
34. El-Asrar AM, Al-Kharashi SA. Full panretinal photocoagulation and early vitrectomy improve prognosis of retinal vasculitis associated with tuberculoprotein hypersensitivity (Eales' disease). Br J Ophthalmol. 2002;86:1248-1251.
35. Kumar A, Tiwari HK, Singh RP, Verma L, Prasad N. Comparative evaluation of early vs.deferred vitrectomy in Eales' disease. Acta Ophthalmol Scand 2000;78:77-78.
36. Shanmugam MP, Badrinath SS, Gopal L, Sharma T. Long term visual results of vitrectomy for Eales disease complications. Int Ophthalmol 1998;22:61-64.
