The current management of post-cataract surgery acute endophthalmitis is greatly influenced by the Endophthalmitis Vitrectomy Study (EVS). The EVS was a multicentric randomized clinical trial that had asked two important questions: (1) Is vitrectomy necessary in all cases of post intraocular lens (IOL) acute bacterial endophthalmitis? and (2) Does systemic antibiotics help in these cases? The EVS recruited 420 patients with acute bacterial post IOL endophthalmitis in 24 centers in the USA. The study recommended that while intravitreal antibiotics must be given to all eyes, vitrectomy should be reserved for eyes with worse presenting vision (light perception, LP, only) and the study did not find any additional benefit of intravenous antibiotics. [1] The major findings of the EVS are listed in Table 1. Are these recommendations followed strictly a decade and half after its first publication in 1995?
We will discuss in this article the management strategies we currently practice at the L V Prasad Eye Institute vis-à-vis the EVS recommendations. These discussions are confined to management of acute post cataract surgery endophthalmitis alone and are based on the evidences generated in our institute over two decades. We will present them in the following seven major headings and finally sum up with the treatment algorithm. (I) Clinical Features; (II) Microbiology; (III) Choice of Intravitreal antibiotics; (IV) Rationale of Intravitreal corticosteroid; (V) Need for systemic antibiotics; and (VI) Cost effectiveness of vitrectomy, (VII) Risk factors, and (VIII) Treatment algorithm.
Table 1 EVS. Design and Recommendations
|
Enrollment |
|
|
Study Design |
|
|
Study Drugs |
|
|
Recommendations |
|
1. Clinical Features
Acute endophthalmitis manifests typically with acute loss of vision associated with pain, redness, lid edema, marked anterior chamber inflammation, anterior chamber fibrin/ hypopyon and vitreous inflammation. Chronic endophthalmitis typically has an indolent course and progressive inflammation. In the EVS visual acuity was less than 5/200 in 90% patients, LP in 26% patients, 86% patients had hypopyon and 25% patients did not have any pain. In our own study 91% patients had vision less than 5/200, 62% patients had LP, 76% patients had hypopyon and 25% patients did not have associated pain. We and others have attempted clinical grading of endophthalmitis related inflammation to quantify endophthalmitis severity. [2,3]
This could be used to possibly differentiate infective endophthalmitis from the toxic anterior segment syndrome (TASS). The principal differentiating features of infection from TASS are listed in Table 2. TASS can improve in time without a special treatment, whereas a diagnosis of endophthalmitis must be made as soon as possible in order to potentially achieve a good result.
The most confirmatory differentiating feature of TASS is the dramatic response to topical corticosteroids. Applied very frequently (every half hour to one hour) TASS eyes respond well to topical corticosteroids in 24 hours. In contrast frequent topical corticosteroids can only temporarily suppress inflammatory component in infective endophthalmitis. Despite these differentiating features it is unwise to take TASS lightly. Until a therapeutic response to topical corticosteroids is clearly present for several days, endophthalmitis should be the prime suspicion. We have earlier reported a series of such cases, and for lack of proper terminology, we had named it “Presumed non-infectious endophthalmitis”. In these series 23 of 27 eyes responded favorably medical therapy alone. [4] The medical therapy consisted of topical corticosteroids in all, topical and systemic antibiotics in 6 patients each (22.2%), and systemic corticosteroid in 12 patients (44.4%).
Table 2Differentiating Features between TASS and Infective Endophthalmitis
|
Features |
TASS |
Infective Endophthalmitis |
|
Timing of Disease |
|
|
|
Pain |
|
|
|
Lid edema |
|
|
|
Conj congestion |
|
|
|
Corneal Edema |
|
|
|
Iris |
|
|
|
Intraocular Pressure |
|
|
|
Therapeutic response to topical corticosteroid |
|
|
2. Microbiology
The final confirmation of infection is obtained from the microbiology laboratory. Undiluted vitreous from an infected eye is the source for microbiology studies. The anterior chamber fluid is not as good a source as the undiluted vitreous. Microscopy uses a variety of stains such as Gram, Giemsa and calcoflur white. The culture media include blood agar, chocolate agar, thioglycollate broth and brain heart infusion for bacteria and potato dextrose agar and Sabouraud dextrose agar for fungi. While the culture is more confirmatory, microscopy of the undiluted vitreous should not be discounted for the simple reason that the initial treatment is either empirical or is based on the vitreous smear report; the culture report is available usually day later. We have reported that more than six polymorphs per high power field are indicative of infection. [5]
The distribution of infecting organism in the EVS and two time period (1991-1997 and 1999-2008) in the L V Prasad Eye Institute is listed in Table 3. There was no fungus in the EVS because the study was confined to bacterial endophthalmitis.
Table 3Microbiology in Endophthalmitis. EVS Vs LVPEI
|
|
EVS [6] |
LVPEI [7] |
LVPEI (unpublished) |
|
90% |
47% |
47.5% |
|
nil |
6% |
10.5% |
|
6% |
26% |
24% |
|
4% |
12.5% |
|
|
nil |
17% (mostlyAspergillusspp.) |
15% (mostlyAspergillusspp.) |
3. Choice of Intravitreal Antibiotics
Intravitreal antibiotics are the main stay of treatment in infective endophthalmitis. While the correct choice of antibiotic is dependent on the culture and sensitivity pattern of the infecting organism, the results of these tests are usually available only days later. Hence the current standard of care is to inject two antibiotics, one against gram-positive organisms and the other against gram-negative organisms. The current initial choice of intravitreal antibiotics in bacterial endophthalmitis, derived from the EVS, are combination of ceftazidime (2.25 mg in 0.1 ml) and vancomycin (1.0 mg in 0.1 ml). The EVS study drugs were amikacin and vancomycin though the former was not advised because of known aminoglycoside toxicity [8] In our own study (1991-1997) [7] the sensitivity pattern of gram-positive organisms were as follows: ciprofloxacin-86%, vancomycin- 84%, cefazoline- 83%; the sensitivity pattern of gram-negative bacteria was as follows: ciprofloxacin- 87%, amikacin-82%, and ceftazidime- 61%. The later decade (1999-2008) (unpublished data) sensitivity pattern is as follows for gram-positive cocci: vancomycin 99%, oflaxacin 95%, and cefazoline 94%; the sensitivity pattern of gram-negative bacilli is as follows: ofloxacin-75%, moxifloxacin-69%, and gatifloxacin 67%.
4. The rationale of Intravitreal Corticosteroid
Intravitreal corticosteroid was not used in the EVS. The EVS recommended a short course of oral corticosteroid (1 mg/KG body weight) a day after the intravitreal antibiotic injection. Endophthalmitis has two components, infection and inflammation. We strongly feel that while intravitreal antibiotics taka care of the infection, intravitreal corticosteroid given concurrently with intravitreal antibiotics takes care of inflammation. [9] In a prospective randomized clinical trial we have demonstrated that intravitreal dexamethasone (400 µg) is useful in controlling inflammation faster without affecting the final visual recovery at three months. [2] We have also shown that intravitreal triamcinolone is useful in very severe inflammation should the infecting organism is also sensitive to the injected antibiotic. [10] Intravitreal and systemic corticosteroids are better avoided in fungal endophthalmitis.
5. Need for Systemic Antibiotics.
Systemic antibiotics used to be a standard of care in treatment of infective endophthalmitis. This went out of favor because the antibiotics used would not cross the blood retinal barrier. This was further substantiated by the EVS. The EVS used intravenous ceftazidime and amikacin, and both of them have poor intraocular penetration. Since then there are several new antibiotics, particularly the fluoroquinolones; they are known to cross the blood retinal barrier in human eyes. [11.12] In our laboratory we have shown that the vitreous concentration of ciprofloxacin following three day oral therapy at 1500 mg/day is 0.66µg/ml in a proven endophthalmitis patient (unpublished data). This vitreous level of ciprofloxacin is more than the minimum inhibitory concentration (MIC) 90 of many common infecting microorganisms. [13] We have also demonstrated that the vitreous vancomycin concentration reduces below MIC 90 in two to three days after the intravitreal injection. [14] This is true for all other intravitreal antibiotics.
The EVS has shown that 9% of the study patients required repeat therapy for non-resolving inflammation /infection [1] and 21% of the repeat culture grew gram-negative microorganisms. These points to the fact that there are a group of patients who need prolonged therapy. Possibly these patients have an infection with more virulent microorganisms. This therapy could be either a repeat intravitreal injection of the culture and sensitivity- adjusted antibiotic or a systemic antibiotic that will rise above MIC 90 in a short period of time, and either act synergistically with the earlier injected intravitreal antibiotic or independently. Hence we recommend use of selective systemic antibiotic in selected cases of endophthalmitis, typically more fulminant endophthalmitis, presumably caused by more virulent microorganisms. Since oral therapy is easy to administer and does not require prolonged hospitalization use of oral ciprofloxacin or gatifloxacin could be beneficial.
6. Cost-effectiveness of Vitrectomy.
Vitrectomy in endophthalmitis helps clear the media, debulks the vitreous and helps superior drug penetration. The EVS did not find added advantage of vitrectomy in eye with vision better than hand motions (HM). The EVS recommended vitreous biopsy only for eye with HM and vitrectomy for eyes with presenting vision of light perception. In our investigation vitrectomy was found superior to vitreous biopsy for an anticipated outcome of 20/40 irrespective the presenting vision was LP or HM. (unpublished). Presuming that any individual with better seeing- eye has better quality of life and could be more productive, probably vitrectomy is as cost-effective as the vitreous biopsy in a given case of endophthalmitis.
7. Risk Factors
The EVS identified eleven risk factors in post-cataract surgery endophthalmitis. The important ones are the corneal infiltrates; cataract wound abnormalities, afferent papillary defect, loss of red reflex, LP vision at presentation, and early onset (within two days of surgery). [1] In addition, we reported that presence of vitreous membrane and exudates (seen ophthalmoscopically or by ultrasonography) is an important risk factor. [15] Presenting vision of light perception had an odds 5.5 for the final visual acuity of <20/60 and presence of vitreous membrane and exudates had an odds of 2.5 for the final visual acuity of <20/400 in our patients.
8. Treatment Algorithm
Our proposed treatment algorithm in post operative acute endophthalmitis management is based on our experience and evidence produced in over two decades. [16] We agree with the EVS recommendation that all patients need intravitreal antibiotics. Vitreous biopsy as recommended by the EVS could probably suffice in patients with visual acuity of 20/400 or even 20/200, not HM. Microbiology of vitreous is mandatory in all patients. We suggest intravitreal corticosteroid (dexamethasone) in all bacterial endophthalmitis. We also suggest oral ciprofloxacin or gatifloxacin in fulminant infection in adults; ciprofloxacin should be the antibiotic of choice inPseudomonasinfection since the MIC 90 of this antibiotic is much lower than gatifloxacin. We suggest that all eyes receive frequent topical antibiotic
(fluoroquinolone), frequent topical corticosteroid (Prednisolone acetate) and cycloplegic (Homide). Should the eye not respond favorably in three days we suggest vitrectomy for eyes initially treated with vitreous biopsy and vitreous biopsy for eyes that had initially had received vitrectomy; in either situation a repeat microbiology and a repeat intravitreal antibiotics are mandatory. Should the eye still not respond in another three days we suggest another intravitreal antibiotic, but now culture and sensitivity adjusted antibiotic. [Figure 1]
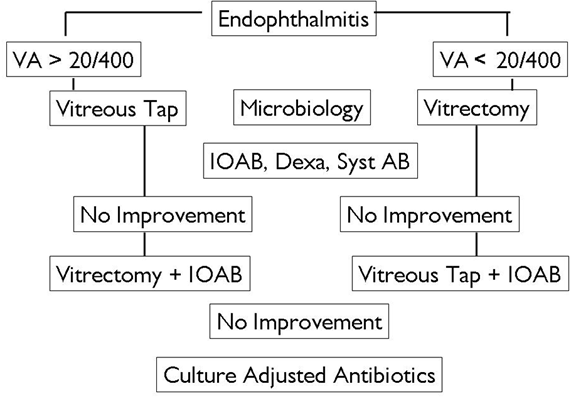
Figure1. Treatment algorithm in infective bacterial endophthalmitis
Using this algorithm we have reported that 20% of our patients gained 20/40 or better, 27% patients gained 20/60 or better, 48% patients gained 20/100 or better, 59% gained 20/200 or better and 61% gained 20/400 or better. [15] This was obviously much less than the EVS outcome of 50% patients gaining 20/40 or better and 75% gaining 20/100 or better. It was because of more virulent infection in our consecutive cases and fungal endophthalmitis in our series. With similar treatment, eyes withStaphylococcus epidermidisinfection have milder presentation and better outcome compared to eyes with fungal infection orPseudomonas aeruginosainfection. [Figure 2-4]
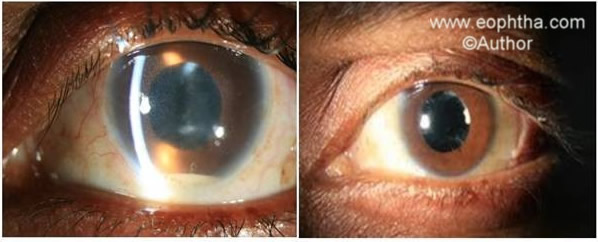
Figure 2. A 45 year old male presented with gross reduction of vision 3 days after cataract surgery. The eye had minimal conjunctival congestion, no pain, hypopyon and cells/flare in the anterior chamber (Left) The vitreous grew Staphyloccous epidermidis. Six weeks following vitreous biopsy, intravitreal antibiotics (cefta and vanco), dexamethasone, and systemic ciprofloxacin the eye was quiet (Right) and vision improved from 20/200 to 20/25
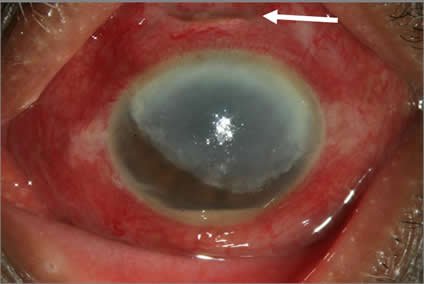
Figure 3. A 67 year old lady presented with gross reduction of vision 30 days after cataract surgery. There was wound gap (white arrow), very congested conjunctiva, three fourth of cornea had a translucent membrane behind and hypopyon. The vitreous grew Aspergillus terreus. Despite vitrectomy, intravitreal amphotericin B and oral ketoconazole the eye could not be salvaged.
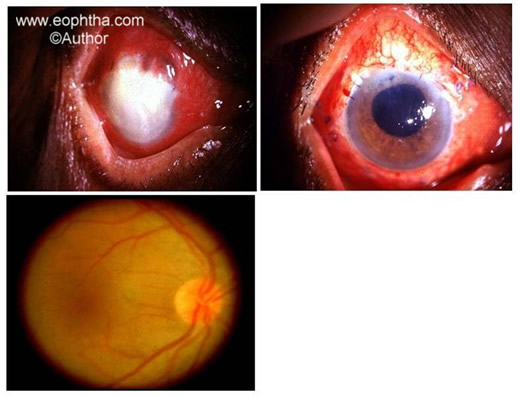
Figure 4. A 77 year old male presented a day after exracapsular cataract and IOL surgery with acute pain and loss of vision in the operated eye. The anterior chamber was full with exudates and there was no view of any other ocular structure (Top Left). Six weeks after IOL explantation, vitrectomy, intraocular antibiotics (amikacin and vancomycin), intraocular dexamethasone, oral ciprofloxacin the eye was quieter and different parts of the eye were visible (Top right). The vitreous grew Pseudomonas aeruginosa. Twelve weeks later the vitreous was clear enough to regain from LP to 20/70 (Bottom).
In last fifteen years numerous advances have been made in treatment of endophthalmitis. Currently we have newer topical and systemic antibiotics that penetrate the blood retinal barrier, we have more potential corticosteroid to control inflammation, we have refined the vitrectomy technology to intervene when necessary, and most importantly we have now newer evidences of endophthalmitis care. The EVS provided us the first such evidence and stimulated the treating surgeons to question the convention. The EVS recommendations continue to be true in a broader sense. We have to only reapply the recommendations in light of the new evidence and local variations.
References
- Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 1995;113:1479–1496
- Das T, Jalali S, Gothwal VK, Sharma S, Naduvilath TJ. Intravitreal dexamethasone in exogenous bacterial endophthalmitis. Results of a prospective randomised study. Br J Ophthalmol. 1999; 83: 1050-1055.
- Meredith TA, Aguilar E#, Miller MJ et al. Comparative treatment of experimental Staphylococcus epidermidis endophthalmitis: a review. Arch Ophthalmol 1990; 108: 857-60.
- Jalali S, Das T, Gupta S. Presumed non-infectious endophthalmitis after cataract surgery. J Cat Refract Surg 1996; 22: 1492-97.
- Sharma S, Jalali S, Adiraju MV, Gopinthan U, Das T, Sensitivity and Predictability of Vitreous Cytology, Biopsy and Membrane Filter Culture in Endophthalmitis. Retina 1996; 16: 525-29.
- Han DP, Wisneiewski SR, Wilson LA et al. Spectrum and susceptibility of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol 1996; 122: 1-17
- Kunimoto DY, Das T, Sharma S, Jalai S, Majji AB, Gopinathan U, Athmanathan S, Rao TN. Microbial spectrum and susceptibility of isolates. Part I. Post- operative endophthalmitis. Am J Ophthalmol. 1999; 128: 240-242.
- Campochiaro PA, Conway BP. Aminoglycoside toxicity: A survey of retina specialists: Implication of ocular use. Arch Ophthalmol 1991; 109: 946-50.
- Das T, Dogra MR , Gopal L, et al. Post surgical endophthalmitis: Diagnosis and management . Indian J Ophthalmol 1995; 43:103- 16.
- Pathengay A, Shah GY, Das T, Sharma S. Intravitreal triamcinolone acetonide in the management of exogenous bacterial endophthalmitis. Am J Ophthalmol 2006; 141: 938-40.
- Lesk MR, Ammann H, Marcil G, et al. The penetration of oral ciprofloxacin into aqueous humor, vitreous, and subretnal fluid of humans. Am J Ophthalmol 1993; 115: 623-28.
- Hariprasad SM, Mieler WF, Holz ER. Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch Ophthalmol 2004;121:345–350.
- Hariprasad SM, Mieler WF. Evidence based medicine and treatment of endophthalmitis in Kertes PJ, Johnson TM (eds) Evidence based eye care. Lippincott, Philadelphia 2007; pp 224-33.
- Raju B, Thiagrajan G, Das T. Modified high performance liquid chromotagraphy (HPLC) technique for detection of vancomycin in human vitreous. Ophthalmic Research 2004; 36: 55-61.
- Das T, Kunimoto DY, Sharma S, et al. Relationship between clinical presentation and visual outcome in postoperative and posttraumatic endophthalmitis in south central India. Indian J Ophthalmol 2005;53:5–16
- Das T, Sharma S. Current management strategies of acute postoperative endophthalmitis. Seminars in Ophthalmology 2003; 18: 109-115
