Introduction
Retinopathy of Prematurity (ROP) is a fibrovascular proliferative disorder, which affects the developing peripheral retinal vasculature of premature infants. It is an avoidable cause of blindness in children.
The initial signs of ROP are detectable by a few weeks after birth, and the condition progresses rapidly thereafter. This means that screening has to be timely, and there is only a very narrow window of opportunity for treating. If not treated, the condition progresses rapidly to Stage 4 or 5 in approximately 50% of babies. The visual prognosis for babies with Stage 5 disease (total retinal detachment) is very poor, even after complex vitreoretinal surgery. The primary goal of screening is to detect all babies with treatable disease in time for treatment to be effective.
Pathogenesis
In the normal foetus, vascular development of the retina occurs in two phases.
Phase 1 (True vasculogenesis):
It occurs from 8-21 weeks of foetal development. Spindle cells (mesenchymal precursor cells) appear around optic disc region. Then cords of spindle cells advance towards ora serrata which differentiate into capillaries which subsequently develop into arterioles and venules. Phase 1 is not under the control of Vascular Endothelial Growth Factor (VEGF)
Phase 2 (Angiogenesis):
Occurs from 22 to 40 weeks of development. Proliferating endothelial cells migrate from existing blood vessels to form new capillaries. Phase 2 is VEGF dependant
Angiogenesis are of 2 types:
Physiologic Angiogenesis:As vascularization is incomplete at birth in preterm infants, the avascular anterior retina causes physiologic hypoxia & VEGF release. Thus immature vessels grow normally
Pathologic Angiogenesis (ROP pathogenesis): When child is exposed to high oxygen after birth there is down regulation of VEGF. This leads to vaso-obliteration & cessation of vessel growth. When oxygen exposure is reduced there is a pathological release of VEGF from now larger avascular retina that leads to neovascularization
Thus, changes in local tissue oxygen level are believed to be important to normal retinal vascular development through the effect of growth factors. If VEGF production persists then the ROP will progress. But if VEGF levels decrease then regression of ROP can occur
Risk factors
Three crucial risk factors:
- Birth weight
- Gestational age
- Number of days oxygen administered
No consensus is present on which of these criteria should be used, either alone or in combination for the screening of ROP.
Other risk factors:
- Multiple births
- Blood transfusions
- Respiratory Distress Syndrome (RDS)
- Sepsis
- Intra Ventricular Hemorrhage (IVH)
- Intra Uterine Growth Retardation (IUGR)
- Vit E deficiency
- Anemia
- Seizures.
Screening
When to screen? When should a pediatrician refer to the ophthalmologist for ROP screening?
Ideally, babies are to be screened at 31 weeks postconceptional age (gestational age + post natal age) or 4 weeks after birth, whichever is later.1 However, an easier way to remember is that thefirst retinal examinationshould be doneby first monthof life.
Whom to screen?
Screening all premature babies will be a waste of time, as we know that all do not develop ROP. UK guidelines state that babies with gestational age (GA) £ 31 weeks or birth weight (BW) £ 1500 g should be screened for ROP. USA guidelines are GA £ 30 weeks or BW £ 1500 g. We cannot follow the western screening guidelines as in the Indian scenario we still see bigger babies getting severe ROP. So, for the Indian scenario all babies having GA £ 35 weeks or having BW £ 1800 g should be screened for ROP. Apart from this, babies that fall outside the screening guidelines but have a rough course in neonatal intensive care unit (NICU) should also be screened at the pediatrician’s discretion. This is called “sickness criteria”.
How to screen?
A retina specialist or a pediatric ophthalmologist does screening. It is done with the help of indirect ophthalmoscope, 28 D lens, scleral depressor (wire vectis) and alphonso speculum. 0.5% proparacaine drops are used for topical anesthesia and half strength tropicamide plus (0.4% tropicamide with 2.5% phenylephrine) is used for pupillary dilatation. Recently, a new digital camera, Retcam, is available for screening but is a very expensive tool. It is the duty of the pediatrician to call these trained ophthalmologists to their NICU or they should refer the children to themat the end of the first month. ROP screening should be included as a part of neonatal care.
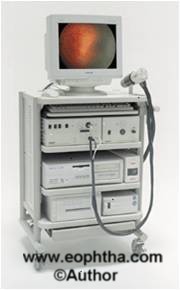
Figure 1:Retcam
Examinations via binocular indirect ophthalmoscope for ROP are difficult and require doctors with specialized paediatric retina training. Moreover hand drawn sketches are the only means of documentation for infant retina, which has its own disadvantages.
RetCam is a digital camera for imaging the retina of infants. It is a mobile self-contained system that can move easily around the hospital or office. It provides state-of the art wide field pediatric retinal imaging (130 degrees). It has instant & accurate documentation, avoiding time-consuming retinal drawings
In just a few minutes one can do an entire exam and the images are stored permanently on a 9.4 GB DVD. It allows easy accessible imaging even by non-ophthalmologists (NICU nurses) and also allows the transmission of these digital images to centers where ROP expertise is available via telemedicine. It provides 24-bit color, mega-pixel digital images, presented on a 17- inch computer monitor available for printout or electronic transmission. Thus it serves as a good teaching tool for others. Comprehensive database keeps track of each image, session and patient allowing for later side-by-side comparison of the case images. FFA can also be done. Newer Retcam II & III have the same features but with a flat screen monitor. Retcam shuttle is laptop based.
Disadvantages of Retcam:Imaging of infant retina till ora in not possible without scleral depression and it is a very expensive tool.
Classification of ROP
An international classification of retinopathy of prematurity was published in 1984 and updated in 1987 and 2005.2-4 The components of classification is as follows.
Location of disease:Each eye is divided in three zones to define the exact location.
- Zone I - circle, the radius of which extends from the disc to twice the distance from the disc to the fovea.
- Zone II – extends from the edge of zone I peripherally to ora serrata nasal and equivalent area near the temporal equator
- Zone III – residual crescent of retina anterior to zone II temporally
The extent of disease:In the form of no. of clock hours involvement (Figure 2)
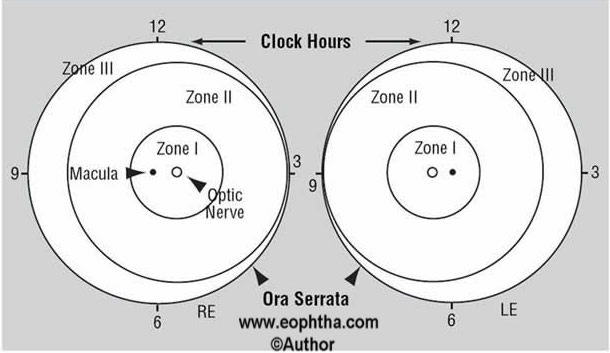
Figure 2: Schematic diagram of right eye (RE) and left eye (LE) showing zones to describe location of disease and clock hours to describe extent of ROP.
Staging of disease:
Staging of the disease is done according to degree of vascular changes. Each stage is defined by its location in zone & extent in clock hours for documentation.
Stage 1 – Demarcation Line:This line is a thin but definite structure that separates the avascular retina anteriorly from the vascularized retina posteriorly. The demarcation line is relatively flat, white, and lies within the plane of the retina (Figure 3).
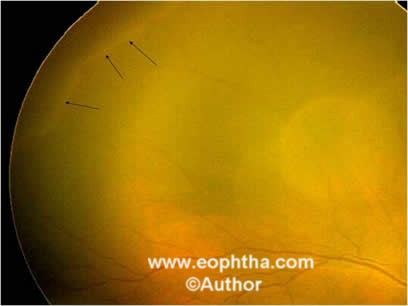
Figure 3: Retcam picture of RE showing stage 1 demarcation line (black arrows).
Stage 2 – Ridge:The ridge is the hallmark of stage 2 ROP. It arises in the region of the demarcation line, has height and width, and extends above the plane of the retina (Figure 4).
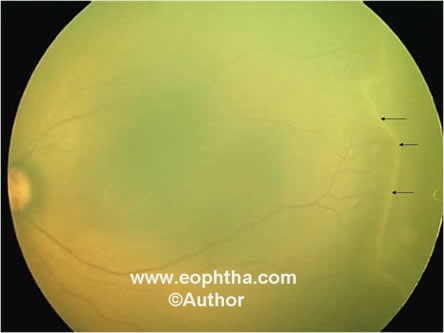
Figure 4: Retcam picture of LE showing stage 2 elevated ridge (black arrows).
Stage 3 – Extraretinal fibrovascular proliferation (EPF):EPF or neovascularization extends from the ridge into the vitreous. It is continuous with the posterior aspect of the ridge (Figure 5). It is further subdivided into mild, moderate or severe depending on the extent of EPF infiltrating the vitreous.
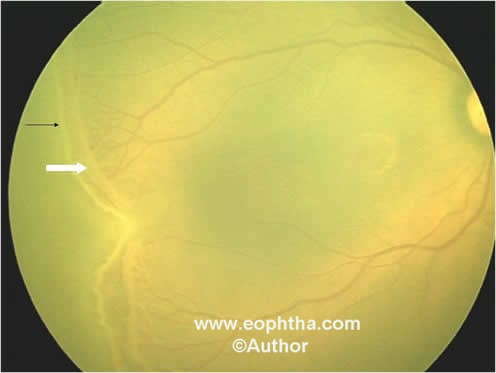
Figure 5: Retcam picture of RE showing stage 3, ridge (black arrow) and extra retinal fibrovascular proliferation (white arrow).
Stage 4 – Partial retinal detachment:Stage 4 is divided into partial retinal detachment not involving fovea, stage 4A (figure 6) and involving fovea, stage 4B. Visual prognosis of stage 4B is poorer than 4A.
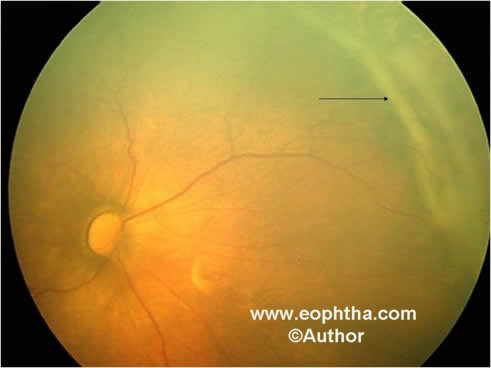
Figure 6: Retcam picture of LE showing stage 4A partial retinal detachment (black arrow) in the temporal periphery not involving fovea.
Stage 5: Total retinal detachment:These retinal detachments are generally tractional and may occasionally be exudative. Visual prognosis is the worst for stage 5 ROP (Figure 7).
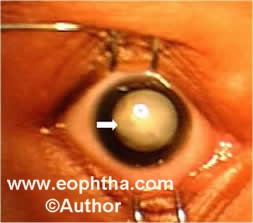
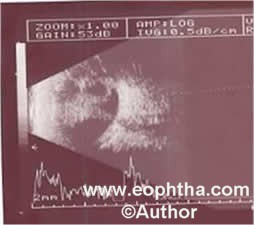
Figure 7: Retcam picture showing leucokoria (white arrow) secondary to total retinal detachment with funnel shaped total retinal detachment on B scan
Figure 8: Fundus picture of LE showing AP-ROP.
Aggressive posterior ROP (AP-ROP):An uncommon, rapidly progressing, severe form of ROP is designated AP-ROP. If untreated, it usually progresses to stage 5 ROP. The characteristic features of AP-ROP are its posterior location, prominence of plus disease, and it does not follow the stages mentioned above(Figure 8). It is observed most commonly in zone I, but may also occur in posterior zone II.
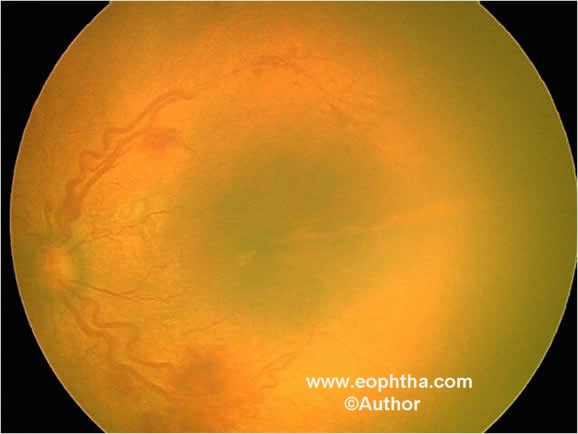
Plus disease:It is an additional sign indicating the severity of active ROP. This includes increased venous dilaattion and arteriolar tortuosity of the posterior retinal vessels (Figure 9) and may later increase in severity to include iris vascular engorgement, poor pupillary dilatation (rigid pupil), and vitreous haze.
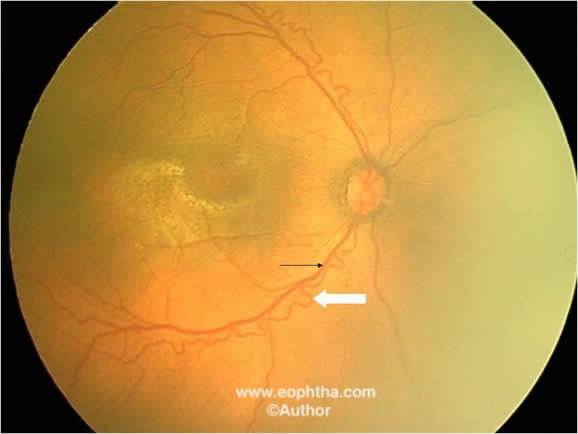
Figure 9: Fundus picture of RE showing venous dilatation (black arrow) and arteriole tortuosity (white arrow) signifying plus disease.
Pre-plus disease:It is defined as vascular abnormalities of the posterior pole that is insufficient for the diagnosis of plus disease but demonstrates more arterial tortuosity and more venous dilatation than normal.
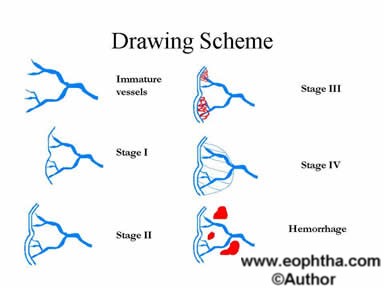
Figure 10: Drawing scheme for ROP.
Management of ROP
When to treat?
- Threshold disease:It was defined by the CRYO-ROP study,5 as Stage 2 in zone I or II involving > 5 contiguous or 8 cumulative clock hours with plus disease. This was the previous “cut off” stage for treatment.
- Prethreshold Disease:Early Treatment ROP (ETROP) study6 has revised the treatment guidelines. This study proved that earlier treatment (Pre Threshold stage) has a better outcome. They divide prethreshold ROP into
- High Risk Prethreshold or Type 1 ROP:This is the new “cut-off” for treatment. It should be treated immediately. It is defined as
- Zone 1 any stage with plus disease or
- Zone 1 stage 3 without plus disease or
- Zone 2 stage 2 or 3 with plus disease.
- Low Risk Prethreshold Disease or Type 2 ROP:These eyes should be considered for treatment only if they progress to type 2 or threshold ROP. It is defined as
- Zone 1 stage 1 or 2 without plus disease or
- Zone 2 stage 3 without the plus disease.
- High Risk Prethreshold or Type 1 ROP:This is the new “cut-off” for treatment. It should be treated immediately. It is defined as
How to treat?
The principle is ablation of the ischemic peripheral retina stops the release of angiogenic factors. Two options are available:
- Cryotherapy:This involves placing a very cool probe on the sclera and freezing until an ice ball is formed on the retina inside. Multiple applications are made to treat the entire avascular retina anterior to the ridge (Figure 11). However, cryotherapy has a lot of disadvantages. It requires general anesthesia, has more local complications like severe lid edema, and for zone I cases, the cryoprobe cannot reach posteriorly because of the restriction caused by the conjunctival fornix.
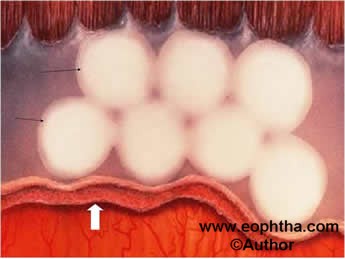
Figure 11: Schematic diagram of fundus showing multiple white cryo burns (black arrows) in avascular retina anterior to ridge (white arrow).
- Laser Photocoagulation:It is a practical alternative after the advent of indirect laser delivery system. Direct treatment of retina from inside, so less local inflammation. The main advantages are that it can be performed under topical anesthesia, systemic and local complications are much less compared to cryotherapy, and it can be done as out patient procedure and posterior retina in zone I cases can be treated easily (Figure 12). Diode red (810 nm) is the laser of choice. However green laser (532 nm) laser can also be used.
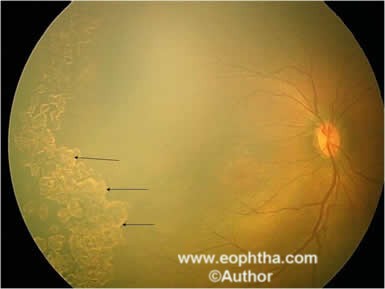
Figure 12: Fundus picture of RE showing laser scars (black arrows).
Laser or cryotherapy can only be done till stage 3 ROP. Management of stages 4 and 5 is the surgical and the final outcome is very poor for these stages.
- Surgical treatment:Surgery is advocated if laser or cryotherapy is unsuccessful in preventing progression to stage 4 or 5. Surgical options available are
- Scleral buckling
- Lens sparing vitrectomy for stage 4
- Lensectomy + vitrectomy
- Open sky vitrectomy for stage 5
In our series7, anatomical success was 90% (9/10) for stage 4A, 44.4% (4/9) for stage 4B, and 14.3% (2/14) for stage 5 ROP. Overall anatomical success was 45.4% (15/33 eyes). Our study has shown poor results as regards to the anatomical outcome following surgery for stages 4 & 5 ROP. This just reemphasizes the need for increasing awareness, having effective screening programmes, timely referral and appropriate intervention.
ROP management doesn’t end with laser or surgery. Once treated, lifelong follow-up (yearly) is mandatory. All other premature infants irrespective of having ROP yearly follow-up till the age of 5 years is advisable to rule out refractive errors (most common), squint, and amblyopia.
Role of anti-VEGF injections in ROP:
- Very controversial
- For normal vascularization of the retina to be completed, VEGF is needed in these premature infants.
- Thus anti-VEGF injections will stop the growth of not only abnormal new vessels but also the normal ones.
- Also, systemic absorption may cause vascular development delay in other organs developing also.
- Currently, anti-VEGF injections are used in ROP only when the standard treatment (which is laser) fails and the disease progresses. It is not recommended as the first line of management.
References
- Reynolds JD, Dobson V, Quine GC, Fielder AR, Palmer EA, Saunders RA, et al, for CRYO-ROP and LIGHT-ROP Cooperative Groups. Evidence based screening criteria for ROP. Natural history data from the CRYO-ROP and LIGHT-ROP studies.Arch Ophthalmol2002;120:1470-76.
- The Committee for the classification of Retinopathy of Prematurity. An international classification for retinopathy of prematurity.Arch Ophthalmol.1984;102:1130-1134.
- ICROP Committee for the classification of late stages of retinopathy of prematurity, II. The classification of retinal detachment. Arch Ophthalmol. 1987;105: 906-912.
- An international committee for the classification of retinopathy of prematurity. The international classification of retinopathy of prematurity revisited.Arch Ophthalmol. 2005;123:991-999.
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trail of cryotherapy for retinopathy of prematurity. Preliminary results.Arch Ophthalmol.1988; 106:471-479.
- Early Treatment of Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity.Arch Ophthalmol2003;121: 1684-96
- Shah PK,Narendran V, Kalpana N, Tawansy KA. Anatomical and visual outcome of stages 4 and 5 retinopathy of prematurity.Eye2009, 23:176-180
