Diabetic maculopathy is the most common cause of visual impairment in patients with diabetes mellitus.
Classification
Diabetic maculopathy is classified as –
- Exudative Maculopathy
- Ischemic Maculopathy
- Mixed Maculopathy
1. Exudative Maculopathy (Diabetic Macular Edema)
It is diagnosed stereoscopically as retinal thickening within 1 disc diameter of the center of the macula using fundus biomicroscopy. To characterize the severity of macular edema and for treatment guidelines, the termclinically significant macular edema (CSME)is used. Macular edema is clinically significant if one of the following conditions is present:
- Retinal thickening at or within 500 mm of the center of the macula; and/or
- Hard exudates at or within 500 mm of the center of the macula if associated with thickening of the adjacent retina; and/or
- A zone or zones of retinal thickening 1 disk area in size, at least part of which is within 1 disk diameter of the macular center.
It is more common in type 2 diabetes (Adult Onset). The incidence of macular edema increases significantly with increasing severity of diabetes in both younger onset and older onset diabetic patients as - IDDM - <5 yrs - 0% & > 20 yrs - 32%; NIDDM - < 5yrs - 3% & > 20 yrs - 28%.
Clinically significant macular edema is further classified into focal or diffuse, depending on the leakage pattern seen on the fluorescein angiogram (FA). In focal CSME, discrete points of retinal hyperfluorescence are present on the FA due to focal leakage of microaneurysms. In diffuse DME, areas of diffuse leakage are noted on the FA due to intraretinal leakage from a dilated retinal capillary bed and/or intraretinal microvascular abnormalities (IRMA), and/or (in severe cases) from arterioles and venules without discrete foci of leaking microaneurysms. There may be associated cystoid macular edema (CME). Cystoid macular edema results from a generalized breakdown of the inner BRB with fluid accumulation, primarily in the outer plexiform layer.
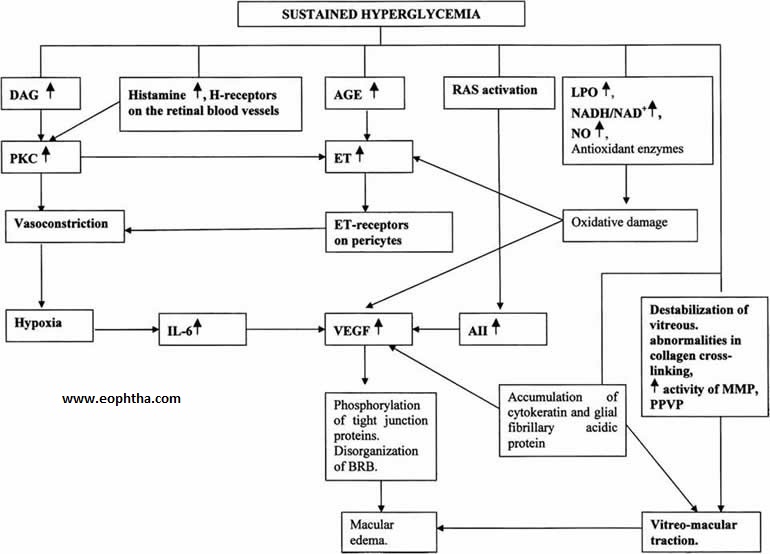
Pathogenesis of diabetic macular edema. AII - angiotensin II; AGE - advanced glycation end products; DAG - diacylglycerol; ET - endothelin; LPO - lypoxygenase; NO - nitric oxide; PKC - protein kinase C; RAS – rennin angiotensin system; VEGF - vascular endothelial growth factor.
2. Ischemic Maculopathy (Diabetic Macular Ischemia, DMI)
This is the most severe form of diabetic maculopathy. Clinically, diabetic macular ischemia is detected by fluorescein angiography as a lack of filling of the macular capillaries. The foveal region in humans is avascular. The foveal avascular zone is polygonal in contour & its size reflects microcapillary foveal circulation. It is enlarged in diabetic ischemic maculopathy. The enlargement results from capillary closure. The contour of FAZ also becomes irregular & notched. There is an increased risk of macular ischemia with diabetic macular edema, increased stage of diabetic retinopathy, and other factors that likely relate to the severity of diabetes, such as the age of onset. Macular non-perfusion has been reported to occur sooner after the diagnosis of type 2 diabetes than type 1 diabetes. The higher prevalence of coexisting systemic hypertension in patients with type 2 diabetes may offer an explanation of this finding.
3.Mixed Maculopathy
It has characteristics of both exudative & ischemic.
.jpg) |
.jpg) |
Imaging Modalities:
1.Fluorescein Angiography
Fluorescein angiography is a standard method used to evaluate patients with DME that is sensitive for qualitative detection of fluid leakage. Leakage on the FA does not equate to clinical retinal thickening or edema. Once a patient is diagnosed with CSME, an angiogram is usually performed to identify the treatable leaking lesions and to evaluate ischemic areas. Ischemic maculopathy is diagnosed when capillary non-perfusion is seen on the FA. Kang et al categorized fluorescein angiographic leakage in DME into three different types: 1) focal leakage: well-defined focal area of leakage from microaneurysms or dilated capillaries; 2) diffuse leakage: presence of widespread leakage from IRMA, retinal capillary bed; 3) diffuse cystoid leakage: diffuse leakage and pooling of dye in the cystic spaces of the macula in the late phase of the angiogram.
Fluorescein angiography (FA) is the gold standard in the clinical diagnosis of diabetic macular ischemia. FA displays relative hypofluorescent areas of retina where there is absence of blood flow through the macular capillaries. At the edges of ischemic retina, associated angiographic findings include arteriolar changes and capillary dilatation. The hallmark findings include enlargement and irregularity of the foveal avascular zone (FAZ) and widened, non-uniform spaces between macular capillaries indicative of intervening capillary loss in the late phase of the fluorescein angiogram, the relative hypofluorescent patches of ischemia are surrounded by hyperfluorescent leakage.
2.Optical Coherence Tomography
OCT has been used for high-resolution imaging of the retina and detection of increased retinal thickness. OCT uses infrared illumination of the fundus to take images and thus is more comfortable and well tolerated by patients than more invasive techniques such as FA.
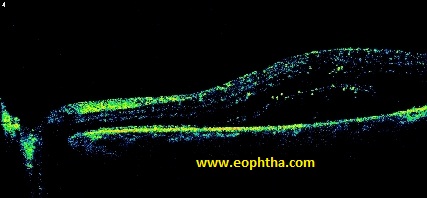
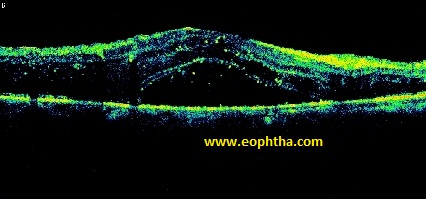
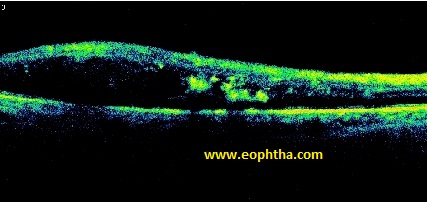
OCT studies of CSME, as defined by the ETDRS, have revealed three basic structural changes in the neurosensory retina: retinal swelling, CME, and serous retinal detachment. Otani et al found that retinal swelling was the most common change in the structure of the retina (88%). The retinal thickness at the central fovea with DME in this study was 250 to 1,000 mm (mean 470 ± 180 mm), whereas normally the retinal thickness with OCT-3 has been determined to be 182 ± 23 mm, slightly more than determined by previous versions of OCT.
Kang et al described four patterns of OCT findings associated with CSME (as defined by the ETDRS): foveal thickening with homogenous optical reflectivity throughout the entire thickness of retina (type 1); foveal thickening with decreased optical reflectivity in the outer layers of the retina (type 2); and foveal thickening with subretinal fluid accumulation with or without retinal traction (types 3A and 3B, respectively). They also showed a significant correlation between these OCT categories and the FA findings: 58% of patients with type 1 pattern OCT had focal leakage on FA, and 92% of patients who had diffuse cystoid leakage on the FA had either a type 2 or type 3A OCT pattern. Moreover, compared to clinical examination, OCT is a more sensitive and specific method for objective evaluation of the attachment of vitreous strands to the edges of fovea and macula and for measurement of macular thickness.
The following morphologic patterns on the basis of OCT examination has been proposed: -
- Diffuse retinal thickening
- Cystoid Macular edema
- Posterior hyaloidal traction
- Sub retinal fluid
- Tractional retinal detachment
OCT has several advantages as a retinal imaging technique: 1) it is non-invasive (no injected dye involved) and well tolerated (especially important in children); 2) it provides quantitative information regarding retinal thickness with a high degree of accuracy and reproducibility; 3) it clearly reveals the presence and extent of vitreomacular traction; and 4) it serves as valuable teaching tool for fellows and residents and is easily understood by most patients. Moreover, OCT can be used to calculate the standardized change in macular thickness (SCMT). As shown by Chan and Duker, SCMT is a highly useful method for evaluation and comparison of the different therapeutic modalities for DME. Disadvantages of currently available OCT machines include the facts that image quality can be affected by media opacities, and the reliability of the data depends on the skill of the OCT operator. Standardized change in macular thickness (SCMT) consists of calculating the actual change in central foveal thickening (the initial pretreatment thickness minus the post-treatment thickness) using OCT and dividing that value by the potential change (the initial pretreatment thickness minus the normal thickness based on normative data) to provide the total improvement in macular edema as a percentage.
SCMT = [actual change]/[potential change] = [initial thickness−final thickness]/ [initial thickness−151 μm] X 100
Not much useful for evaluation of DMI. In the absence of edema, the ischemic areas in the macula appear thin on OCT.
 |
|
 |
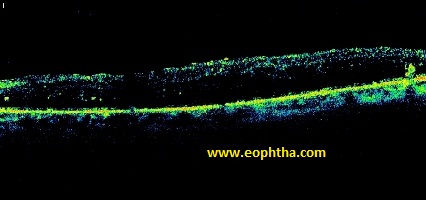
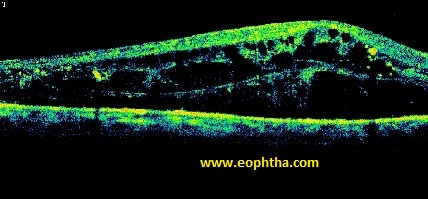 |
Severe Cystoid Edema
 |
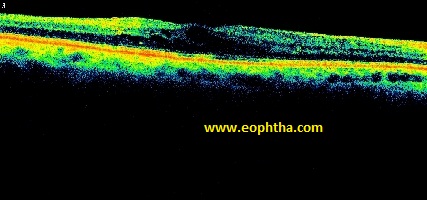 |
|
Spongy Edema |
Mild Cystoid Edema |
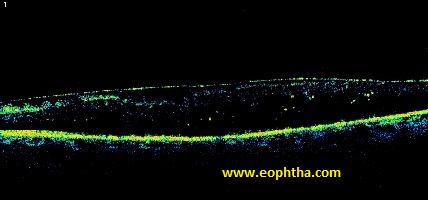
Taut Posterior Hyaloid
Treatment
The treatment of various forms of maculopathy differs. Although there is no established treatment of diabetic macular ischemia, management decisions are complicated by its presence. DMI is important as it relates to visual function. Not surprisingly, diabetic macular ischemia portends a poor prognosis in diabetic retinopathy. Macular ischemia may be present in eyes with potentially treatable macular pathology (Mixed Maculopathy) and may impart a poor response to treatment. The treatment of DME is considered as under -
Laser Photocoagulation
Indications –
Focal leaks > 500 μ from the centre of macula.
Focal leaks 300 – 500 μ from the centre of macula, if the treating ophthalmologist does not believe that the treatment is likely to destroy the remaining perifoveal capillary network.
Areas of diffuse leakage that has not been treated previously.
Avascular zones other than normal FAZ, not previously treated.
Mechanism of action
Reduces metabolic need.
Direct diffusion of oxygen from choriocapillaries thru scar to inner retina.
Stimulates endothelial repair process.
Types
Focal
Classic Grid
Modified Grid
|
Focal |
Classic Grid |
Modified Grid |
|
|
Wavelength |
Argon Green, Double Frequency Nd:YAG, Dye Yellow |
||
|
Duration |
100 ms |
||
|
Spot Size |
50 µ |
50 µ |
|
|
Power |
80 mw (Titrated according to requirement) |
50 mw (Titrated according to requirement) |
|
|
Inter burn distance |
- |
2 burn |
|
|
Burn Type |
Grade 2 - 3 (dirty White)/ Blanching or Darkening of MA |
Grade 1 - 2 (Faint White) |
|
|
Remarks |
Burns are given at 500 µ from the fovea to all MAs within 3000 µ |
Burns are given at 500 µ from the fovea in aCpattern sparing papilla-macular bundle, can extend upto 2 disc diameters from the centre of macula. |
Only thickened areas are treated & not the whole macula, can extend upto 2 disc diameters from the centre of macula. |
|
Follow up |
1 wk, 1 m, 4 m FFA |
1 wk, 1 m, 4 m FFA |
1 wk, 1 m, 4 m FFA |
|
Practical tips for laser photocoagulation for diabetic macular edema - |
In general, the threshold for performing focal/ grid laser photocoagulation occurs when the central subfield mean thickness attains 250 mm, and on average, for this threshold level of edema, one can expect approximately 25 mm of macular thinning after focal/ grid laser at the usual first follow-up interval of 3–4 months. For every 100 mm of additional baseline macular thickening above this 250 mm threshold, one can expect that focal/grid laser will yield approximately 10 mm of additional macular thinning at the 3–4-month follow-up visit.
The natural history of lipid exudates associated with DME is to spontaneously resolve over 2 years or longer. Macrophages clear the exudates by phagocytosis. Both serum lipid control and focal/grid photocoagulation can independently accelerate the clearance of lipid exudates in DME. A potential clinical problem is the subfoveal migration of hard lipid after focal/grid laser treatment, particularly in patients with heavy lipid exudates before treatment. Adjunctive oral statin therapy in such patients has been reported to eliminate this problem in a small clinical trial.
Side effects
-
- Paracentral scotomas
- Immediate risk of vision loss.
Poor Prognostic Factors–
-
- Hard exudates involving the fovea
- Mixed Maculopathy
- Severe retinopathy at presentation
Sub-threshold Micropulse Diode Laser Photocoagulation (SMDLP)
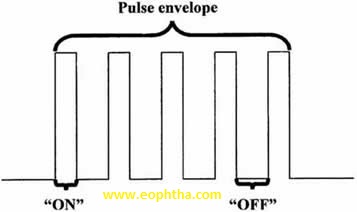
Micropulse laser treatment employs repetitive trains (50–500) of laser pulses of short duration (0.8–5.0 ms). Powers used have varied between 5 and 50 mW and energy per pulse between 70 and 175 mJ. Wavelengths of 514, 524, 527, 532, and 810 nm have been used. Individual microaneurysms are not targeted, in contrast to conventional focal/grid laser treatment. The intent of treatment is to lightly affect the retinal pigment epithelium alone, restricting the lateral spread of heat and thus sparing damage to the overlying photoreceptors and mid-retinal interneurons. The off period between pulses allows time for heat dissipation to achieve this goal. Although attractive as a concept, the practical problem remains that the clinician cannot tell where treatment has been applied, because the lesions are ophthalmoscopically invisible and can only be detected by fluorescein angiography (hyperfluorescence) or autofluorescence photography (hypo-autofluorescence), both cumbersome ways of monitoring the distribution of lesions. Methods of choosing power to use are ad hoc and variable and based on choosing a power that is some fraction of that producing a visible burn in an area remote from the macula. Moreover, the power setting is not changed, regardless of the presence of subretinal fluid or variable macular thickening, a concept that is counterintuitive to the stated goal of producing uniform lesions at the level of the RPE. There are no microscotomata detectable by microperimetry over micropulse laser lesions by 3 months after treatment.
Peribulbar Triamcinolone Injection
Peribulbar triamcinolone or methylprednisolone injections have been used to treat DME either as monotherapy or as adjunctive therapy to focal/grid laser, and short-term efficacy in thinning the macula and improving visual acuity has been demonstrated. Evidence exists that peribulbar triamcinolone is less effective than intravitreal Triamcinolone in improving visual acuity in DME over a 3-month period. In a pilot prospective, randomized, multicentered clinical trial, neither peribulbar monotherapy nor combination therapy showed clinically important superiority over modified ETDRS focal/grid photocoagulation, and, therefore, a large-scale clinical trial was not done. The complications of peribulbar triamcinolone injection include intraocular pressure elevation and ptosis in approximately 10 and 20% of cases, respectively.
Intravitreal Triamcinolone Injection
Jonas and Sofker were the first to use intravitreal triamcinolone injection in the treatment of DME in 2001. In a randomized clinical trial comparing intravitreal triamcinolone injection to observation, the efficacy of triamcinolone injections was established through 2 years of follow-up. Many case series and a separate shorter term placebo controlled randomized trial have supported this conclusion. The benefits of this form of therapy must be balanced by consideration of the side effects including surgical cataract formation in 51% of phakic eyes at 2 years follow-up and intraocular pressure elevation in 25–40% of eyes. In a randomized clinical trial comparing serial intravitreal triamcinolone injection therapy using 1 or 4mg to focal/grid photocoagulation, focal/grid photocoagulation showed superior efficacy and fewer side effects; thus, intravitreal triamcinolone therapy is a second line therapy for consideration when focal photocoagulation has failed. In this study, the mean visual acuity was four–six ETDRS letters better in the laser group than in the 1 and 4 mg intravitreal Triamcinolone groups after 2 years, and the improved visual outcomes persisted after discounting the effects of cataract progression in the triamcinolone groups. The mean central subfield mean thickness decreased 53–62 mm more in the focal/grid group compared to the 1- and 4-mg triamcinolone groups at 2 years, respectively. The 4-mg triamcinolone group was superior to the focal/grid laser group at the 4-month follow-up visit, but that short-term advantage of triamcinolone had vanished by 1 year follow-up, and at 2 years follow-up the focal/grid group had clearly superior outcomes. Many doses of intravitreal triamcinolone have been used, including 1, 2, 4, 5, 8, 13, and 20–25 mg. In general, there is inconclusive but suggestive evidence that duration of action is longer with higher doses and that frequency of intraocular pressure rise increases with higher doses. There is no consistent evidence that higher doses are more effective for thinning the macula or improving visual acuity than lower doses, and some evidence exists that lower doses have greater efficacy for macular thinning than higher doses. In a small pilot randomized trial, intravitreal triamcinolone injection was associated with a higher rate of partial vitreous separation with foveal attachment or traction compared to eyes treated with focal/grid photocoagulation. Eyes with this change had worse visual outcomes.
Intravitreal Dexamethasone Delivery System
A sustained release drug delivery system for dexamethasone inserted transclerally into the vitreous produced statistically significant visual acuity improvement for 90 days after insertion and was well tolerated for 180 days. For the 700 mg device, the probability of a 10 letter improvement in visual acuity at 90 days was 2.8 times greater in the device group compared to the observation group. Mean central retinal thickness decreased by more than 160 mm at 90 days, but this figure included pooled results from other causes of macular edema besides DME. Eleven percent of treated patients had an intraocular pressure rise of 10 mmHg or higher. This device is presently undergoing further clinical trials.
Intravitreal Injections of Anti-VEGF Drugs
Intravitreal injections of anti-VEGF drugs also produce reductions in macular thickening, but on average the magnitudes of the reductions and the durations of responses are less than with intravitreal triamcinolone injections, suggesting that biochemical pathways not involving VEGF are important in the pathogenesis of DME. Intravitreal pegaptanib, bevacizumab, ranibizumab, and VEGF-trap all fall into this category. Counterbalancing their lesser efficacy compared to steroids, the anti-VEGF drugs have a more favorable side effect profile, in particular without the tendency to cause cataract and raise intraocular pressure. Instances have been reported in which intravitreal pegaptanib injected in one eye has been associated with regression in neovascularization in the fellow eye. Similar concerns have been expressed for bevacizumab, but, despite study, no evidence of a fellow eye effect on DME has been found, suggesting that systemic absorption is negligible. Limited data suggest that fears that intravitreal anti-VEGF drugs could worsen macular ischemia are unfounded. No clinically important differences in effects on DME of 1.25 vs. 2.5 mg of bevacizumab have been reported in studies comparing the two. Some evidence suggests that these drugs are more effective in treatment of na?¨ve eyes than in eyes previously treated with focal/grid laser.
DRCR.net is conducting trials comparing Intravitreal anti-VEGF’s (Ranibizumab) Vs Intravitreal Triamcinolone for DME with or without laser. They found that Intravitreal ranibizumab with prompt or deferred laser is more effective through at least 1 year compared with prompt laser alone for the treatment of DME involving the central macula. Ranibizumab should be considered for patients with DME with glaucoma as well as those who are phakic. In pseudophakic eyes, intravitreal triamcinolone prompt laser seems more effective than laser alone but frequently increases the risk of intraocular pressure elevation.
Supplemental Oxygen and Hyperbaric Oxygenation
Hyperbaric oxygenation reduced breakdown of the blood–retinal barrier in a rat model of diabetic retinopathy. In a small clinical series, 10–30 hyperbaric oxygen treatments resulted in no morphologic change in DME. Three months of supplemental oxygen therapy by nasal cannula improved macular thickness in nine eyes of five patients with DME refractory to conventional focal/grid laser treatment. Three eyes showed 2 lines improvement in visual acuity and five of nine eyes worsened after cessation of supplemental oxygen. More experience is needed with these therapies before any recommendations for use can be considered.
Certain other forms of DME
Subclinical Diabetic Macular Edema
Diabetic macular edema may be clinically recognized, yet not reach a severity satisfying the definition of CSME. Clinical assessment of macular edema and OCT assessment of macular edema frequently disagree in this group of patients. In addition, some eyes do not have clinically recognized DME, but macular thickening is detectable by OCT. The term subclinical DME (SCDME) has been used to define both of these classes of DME that are less severe than clinically significant DME.
Refractory Diabetic Macular Edema
Diabetic macular edema that has been treated with focal/grid photocoagulation and yet persists is defined as refractory. Different authors use different criteria for the number of focal/grid treatments that are used before applying the term, but all require at least one. Eyes may be refractory to treatments other than focal/grid photocoagulation and some eyes have edema refractory to all known treatments for DME. Refractory DME has been reported to produce more rapid visual acuity loss in older patients, and is associated with greater short-term variability in macular thickness than is seen in eyes without refractory DME. It is also associated with higher levels of HbA1c than DME that resolves with focal/grid laser photocoagulation.
Regressed Diabetic Macular Edema
It is defined as the macular state in which DME was once present, was treated with focal/grid laser or other treatments, and is now absent. It is important to state the method for determining absence of the macular edema, as classifications may disagree using clinical methods and imaging methods such as OCT or fluorescein angiography.
Recurrent Diabetic Macular Edema
Regardless of the treatment used, cases exist in which DME vanishes after treatment, but subsequently recurs. Although DME can resolve spontaneously without treatment, and then recur, the term recurrent DME is generally used with reference to treated eyes with recurrences.
Future Directions
Although fraught with uncertainty, it is interesting to hazard some predictions about directions of future research into the causes and best management of DME. Genetic mutations rendering patients more or less susceptible to DME as a complication of diabetes are likely to be defined. The physiologic pathways contributing to DME and not mediated by VEGF are a likely focus of future research. Most patients with DME take many medications, and many of them, such as certain classes of antihypertensive drugs, newer oral agents for management of diabetes, and statins, have theoretical bearing on retinal physiology. The interactions of drugs with DME are presently unexplored and should be clarified in the next years. Using SD-OCT, it should be possible to define at almost histologic levels the retinal changes occurring in DME and determine which, if any, changes associate with visual outcomes. Newer methods of laser photocoagulation are likely to become increasingly tested against conventional photocoagulation. The semi-automated production of laser patterns that is currently found in certain commercial laser machines may overcome the problem with micropulse laser of not knowing where treatment has been applied. Refinements in the best methods of application of focal/grid photocoagulation and the role of alternative therapies such as pharmacologic adjuncts, combined therapies, and vitrectomy will likely flow from clinical trials. Trials using visual acuity as the primary endpoint are expensive and lengthy. The quest for surrogate clinical endpoints that correlate well with visual acuity and could allow randomized trials to be simplified and shortened continues.
OCT-measured macular thickness has not been the hoped-for surrogate, but other endpoints, such as retinal vessel diameter, are being evaluated.
References:
- Klein R, Klein B, Moss S, Cruickshanks K. The Wisconsin epidemiologic study of diabetic retinopathy: the long-term incidence of macular edema. Ophthalmology. 1995;102: 7–16.
- Klein R, Klein BE, Moss SE, Linton KL. The Beaver Dam eye study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology. 1992;99:58–62.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy. A systematic review. JAMA. 2007; 298:902–916.
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985;103:1796–1806.
- Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy. The Beijing eye study 2006. Graefe’s Arch Clin Exp Ophthalmol. 2008;246:1519–1526.
- Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, et al. Prevalence and risk factors for diabetic retinopathy. The Singapore Malay eye study. Ophthalmology. 2008;115:1869–75.
- Rubino A, Rousculp MD, Davis K, Wang J, Girach A. Diagnosed diabetic retinopathy in France, Italy, Spain, and the United Kingdom. Prim Care Diabetes. 2007;1:75–80.
- Wong TY, Klein R, Islam FMA, Cotch MF, Folsom AR, Klein BEK, et al. Diabetic retinopathy in a multiethnic cohort in the United States? Am J Ophthalmol. 2006;141:446–455.
- Varma R, Torres M, Pena F, Klein R, Azen SP, Los Angeles Latino eye study group. Prevalence of diabetic retinopathy in adult latinos. Am Acad Ophthalmol. 2004;111:1298–1306.
- Wolfe JA, HortonMB, McAteerMB, Szuter CF, Clayton T. Race, macular degeneration, and diabetic maculopathy. Arch Ophthalmol. 1993;111:1603–1604.
- Shea AM, Curtis LH, Hammill BG, Kowalski JW, Ravelo A, Lee PP, et al. Resource use and costs associated with diabetic macular edema in elderly persons. Arch Ophthalmol. 2008;126:1748–1754.
- Klein BEK, Moss SE, Klein R, Surawicz TS. The Wisconsin epidemiologic study of diabetic retinopathy XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98: 1261–1265.
- Sigelman J. Diabetic macular edema in juvenile and adult onset diabetes. Am J Ophthalmol. 1980;90:287–296.
- Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004; 53:2883–2892.
- Vitale S, Maguire MG, Murphy RP, Hiner CJ, Rourke L, Sackett C, et al. Clinically significant macular edema in type 1 diabetes incidence and risk factors. Ophthalmology. 1995;102:1170–1176.
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BEK. The Wisconsin epidemiologic study of diabetic retinopathy XXIII. The twenty-five year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503.
- Klein R, Klein BE, Moss SE, David MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy IV. Diabetic macular edema. Ophthalmology. 1984; 91:1464–1474.
- Klein R, Klein BEK, Moss SE, Cruickshanks KJ. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–1815.
- Lattanzio R, Brancato R, Pierro L, Bandello F, Iaccheri B, Fiore T, et al. Macular thickness measured by optical coherence tomography (OCT) in diabetic patients. Eur J Ophthalmol. 2002;12:482–487.
- Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2007;115: 533–539.
- Iwasaki M, Inomata H. Relation between superficial capillaries and foveal structures in the human retina. Invest Ophthalmol Vis Sci. 1986;27:1698–1705.
- Riva C, Petrig B. Blue field entoptic phenomenon and blood velocity in the retinal capillaries. Opt Soc Am. 1980;70:1234–1238.
- Henkind P. Symposium on glaucoma: joint meeting with the national society for the prevention of blindness: new observations on the radial peripapillary capillaries. Invest Ophthalmol Vis Sci. 1967;6:103–108.
- Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51: 364–380.
- Moore J, Bagley S, Ireland G, Mcleod D, Boulton ME. Three dimensional analysis of microaneurysms in the human diabetic retina. J Anat. 1999;194:89–100.
- TolentinoMJ,McLeodDS,TaomotoM,OtsujiT,Adamis AP, Lutty GA. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002;133:373–385
- Sachdev N, Gupta V, Abhiramurthy V, Singh R, Gupta A. Correlation between microaneurysm closure rate and reduction in macular thickness following laser photocoagulation of diabetic macular edema. Eye. 2008; 975–977.
- Nishikawa H, Shahidi M, Vitale S, Alexander J, Asrani S, Mori M, et al. Relation between retinal thickening and clinically visible fundus pathologies in mild nonproliferative diabetic retinopathy. Ophthalmic Surg Lasers. 2002; 33:127–134.
- Blair NP, Shahidi M, Lai WW, Zelkha R. Correlation between microaneurysms and retinal thickness in diabetic macular edema. Retina. 2008;28:1097–1103.
- DiabeticRetinopathy Clinical Research Network. Comparison of the modified early treatment diabetic retinopathy study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–480.
- Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88:1060–1063.
- Fryczkowki AW, Hodes BL, Walker J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int Ophthalmol. 1989;13:269–279.
- Quintyn JC, Brasseur G. Subretinal fluid in primary rhegmatogenous retinal detachment: physiopathology and composition. Surv Ophthalmol. 2004;49:96–108.
- LaCour M. The retinal pigment epithelium. In: Kaufman PL, ed. Adler’s Physiology of the Eye: Clinical Application. St. Louis: Mosby; 2003:348–357.
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW, Penn State Retina Research Group. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47:S253–S262.
- Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413.
- Vinores SA, McGehee R, Lee A, Gadegbeku C, Campochiaro P. Ultrastructural localization of blood-retinal barrier breakdown in diabetic and galactosemic rats. J Histochem Cytochem. 1990;38:1341–1352.
- Tso M, Cunha-Vaz J, Shih C, Jones C. Clinicopathologic study of blood-retinal barrier in experimental diabetes mellitus. Arch Ophthalmol. 1980;98:2032–2040.
- Bringmann A, Uckermann O, Pannicke T, Iandiev I, Reichenbach A, Wiedemann P. Neuronal versus glial cell swelling in the ischemic retina. Acta Ophthalmol Scand. 2005;83:528–538.
- Distler C, Dreher Z. Glia cells in the monkey retina-II. Muller cells. Vision Res. 1996;36:2381–2394.
- Antcliff R J, Hussain AA, Marshall J. Hydraulic conductivity of fixed retinal tissue after sequential excimer laser ablation-barriers limiting fluid distribution and implications for cystoid macular edema. Arch Ophthalmol. 2001;119:539–544.
- Sebag J, Buckingham B, Charles A, Reiser K. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch Ophthalmol. 1992;110: 1472–1476.
- Chang YH, Chen PL, Tai MC, Chen CH, Lu DW, Chen JT. Hyperbaric oxygen therapy ameliorates the blood-retinal barrier breakdown in diabetic retinopathy. Clin Exp Ophthalmol. 2006;34:584–589.
- Krott R, Heller R, Aisenbrey S, Bart-Schmidt KU. Adjunctive hyperbaric oxygenation in macular edema of vascular origin. Undersea Hyperb Med. 2000;27: 195–204.
- Nguyen QD, Shah SM, Anden EV, Sung JU, Vitale S, Campochiaro PA. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest Ophthalmol Vis Sci. 2004;45:617–624.
- Sigurdsson R, Begg I. Organised macular plaques in exudative diabetic maculopathy. Br J Ophthalmol. 1980; 64:392–397.
- Avci R, Inan UU, Kaderli B. Long-term results of excision of plaque-like foveal hard exudates in patients with chronic diabetic macular edema. Eye. 2008;22: 1099–1104.
- Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy- ETDRS report number 9. Ophthalmology. 1991;98: 766–785.
- Maia O O Jr,Takahashi B S, Costa R A, Scott I U,Takahashi WY. Combined laser and intravitreal triamcinolone for proliferative diabetic retinopathy and macular edema: one-year results of a randomized clinical trial. Am J Ophthalmol. 2009;147:291–297.
- Mason JO III, Nixon PA, White MF. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142:685–688.
- McDonald HR, Schatz H. Macular edema following panretinal photocoagulation. Retina. 1985;5:5–10.
- McDonald H, Schatz H. Visual loss following panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1985;92:388–393.
- Nonaka A, Kiryu J, Tsujikawa A, Yamashiro K, Nishijima K, Kamizuru H, et al. Inflammatory response after scatter laser photocoagulation in non-photocoagulated retina. Invest Ophthalmol Vis Sci. 2002;43(4):1204–1209.
- Er H, Doganay S, Turkoz Y, Cekmen M, Daglioglu M C, Gunduz A, et al. The levels of cytokines and nitric oxide in rabbit vitreous humor after retinal laser photocoagulation. Ophthalmic Surg Lasers. 2000;31(6):479–483.
- Shimura M, Yasuda K, Nakazawa T, Kano T, Ohta S, Tamai M. Quantifying alterations of macular thickness before and after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Ophthalmology. 2003;110:2386–2394.
- Shimura M, Yasuda K, Nakazawa T, Tamai M. Visual dysfunction after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Am J Ophthalmol. 2005;140:8–15.
- Browning DJ. Visual dysfunction after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Am J Ophthalmol. 2005;140: 127–128.
- Shimura M, Yasuda K, Shiono T. Posterior sub-tenon’s capsule injection of triamcinolone acetonide prevents panretinal photocoagulation-induced visual dysfunction in patients with severe diabetic retinopathy and good vision. Ophthalmology. 2006;113:381–387.
- Mason JO III, Yunker JJ, Vail R, McGwin G Jr. Intravitreal bevacizumab (Avastin) prevention of panretinal photocoagulation-induced complications in patients with severe proliferative diabetic retinopathy. Retina. 2008;28:1319–1324.
- Blankenship G. A clinical comparison of central and peripheral argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1988;95:170–177.
- Diabetic Retinopathy Clinical Research Network. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127:132–140.
- Chung EJ, Roh MI, Kwon OW, Koh HJ. Effects of macular ischemia on the outcome of intravitreal bevacizumab therapy for diabetic macular edema. Retina. 2008;28:957–963.
- Yang CM. Surgical treatment for severe diabetic macular edema with massive hard exudates? Retina. 2000; 20:121–125.
- Kang SW, Park SC, Cho HY, Kang JH. Triple therapy of vitrectomy, intravitreal triamcinolone, and macular laser photocoagulation for intractable diabetic macular edema. Am J Ophthalmol. 2007;144:878–885.