1. Type of macular neovascularisation (MNV):
Macular neovascularisation (MNV) is a broad disease entity characterised by the development of the abnormal vessels arising either from the retinal vasculature (deep capillary plexus) or choroidal vasculature (choriocapillaris). Choroidal neovascular membrane (CNVM) is a subtype of MNV, characterised by the abnormal growths of blood vessels from the choroidal capillaries that penetrate the retina, specifically into the sub-RPE and subretinal spaces. These vessels are extremely immature and extremely fragile, so they bleed and leak, de-structuring the macula, causing severe visual impairment.
The classification of MNV is depicted below in Figure 1:
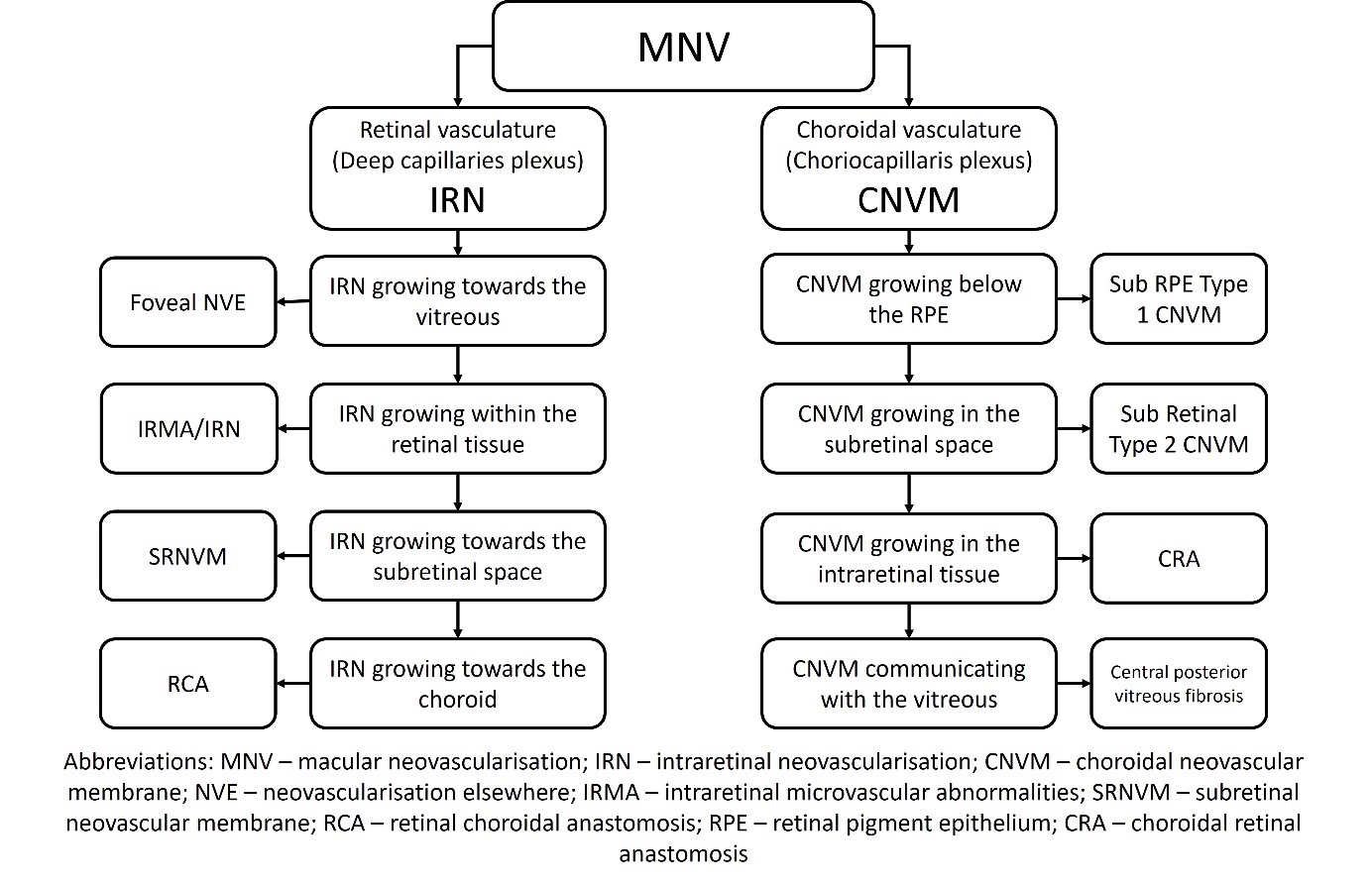
2. Cause of CNVM:
A CNVM is primarily classified based on the etiology into two categories: A) Age-related Macular Degeneration (AMD) associated CNVM and B) Non-AMD associated CNVMs.
Causes of non-AMD associated CNVMs:
- Central serous chorioretinopathy
- Pachychoroid neovasculopathy
- Choroidal rupture in blunt trauma
- Pathological myopia
- Angioid streaks
- Choroidal nevus
- Posterior uveitis (inflammatory CNVM)
- Ocular histoplasmosis syndrome
- Scar-related CNVM
- Tumours like choroidal osteoma
- Iatrogenic CNVM
- Idiopathic CNVM
AMD associated CNVMs are usually either type 1 sub RPE or type 2 M (mixed type 1 and 2 patterns) on OCT. On the other hand, non-AMD associated CNVMs are either purely type 1 or type 2 in location on OCT.
3. Types of CNVM on OCT based on its location:
CNVMs can be identified at four levels on OCT:
Type 1 – Below the RPE (sub-RPE)
Type 2 – Above the RPE in neurosensory space (sub-retinal)
Type 2M – Mixed patterns present both in the sub-RPE and subretinal spaces
Type 3 – Neovascularisation present arising from the retinal capillaries
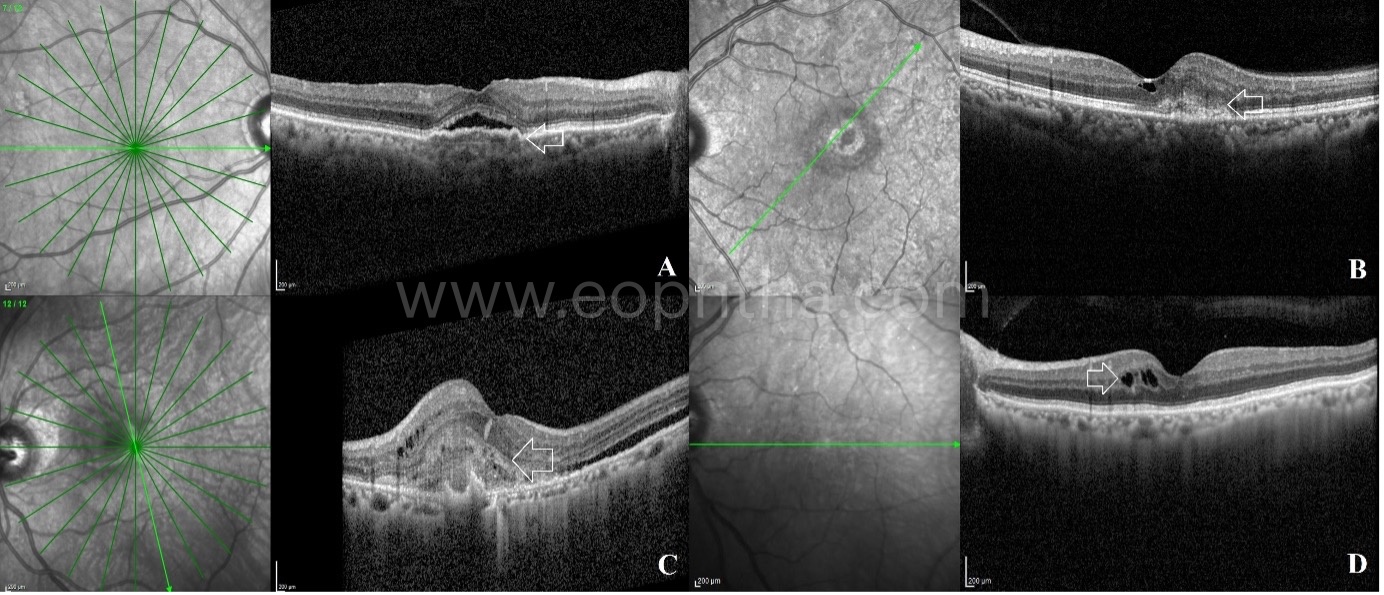
4. Location of the CNVM in relation to fovea:
On the basis of the relationship of the location of CNVM with the fovea; the CNVMs are classified into 3 categories:
Sub foveal CNVM – when the CNVM is located directly underneath the fovea
Juxta foveal CNVM – when the CNVM is close to the fovea but not involving the foveal center
Extra foveal CNVM – when the CNVM is located away from the fovea.
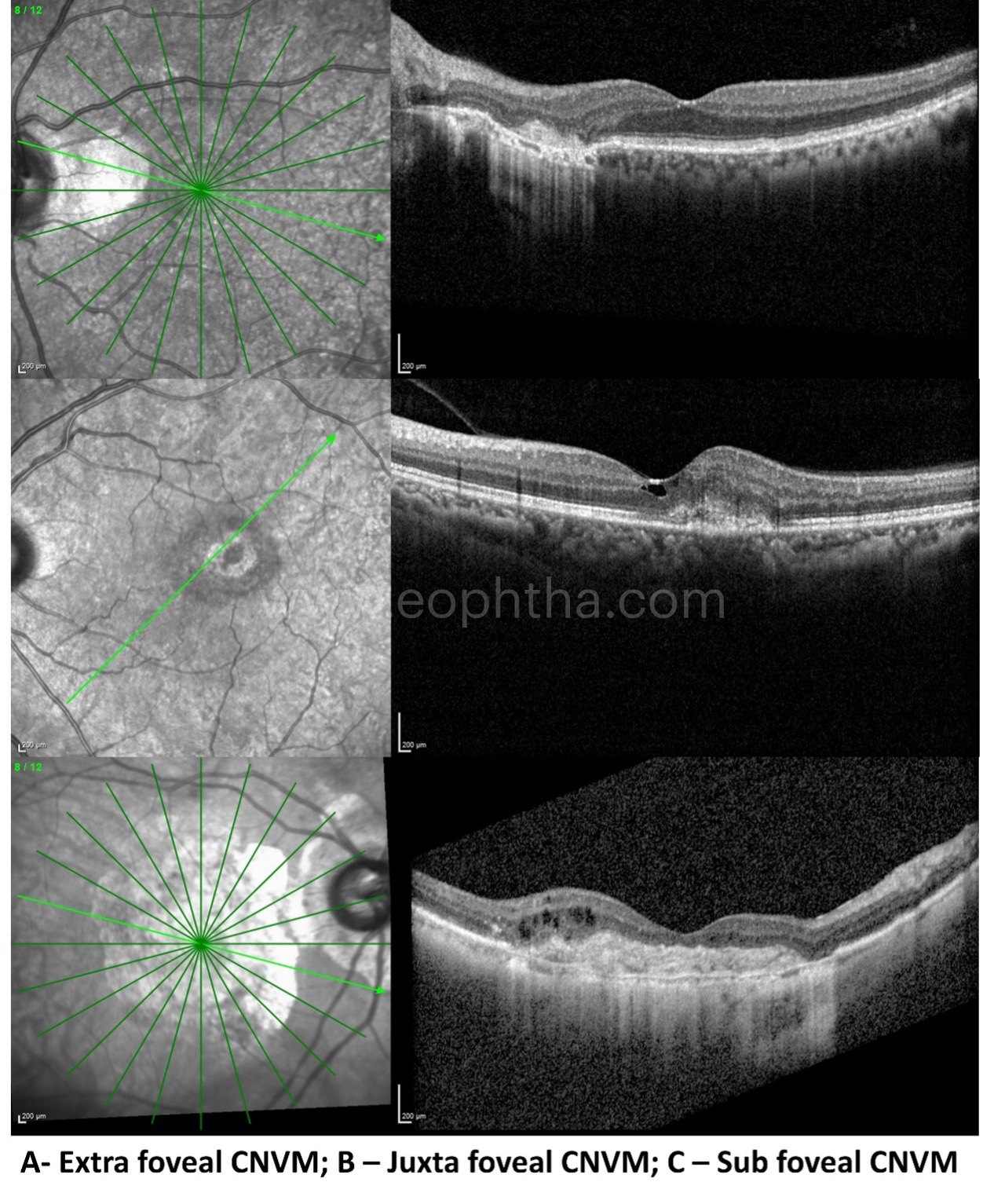
Clinical relevance of CNVM location:
Sub foveal CNVM (especially type 2) associated with poor visual prognosis
Sub foveal type 1 CNVM associated with excellent visual prognosis
Juxta foveal and extrafoveal CNVM (type 1 and 2) associated with good VA
5. CNVM Activity:
An active CNVM is defined by the ability of the neovascular membrane to leak blood, fluid and/or exudates into the extravascular space. Three compartments are identified where the leakage from the CNVM can accumulate or aggregate. These include the sub RPE space, the subretinal space and the intraretinal space.
Thus, the different OCT features which signify activity from a CNVM include:
Leakage of fluid
- Serous/serosanguinous PED
- Subretinal fluid
- Intraretinal fluid
Leakage of heme
- Sub RPE hemorrhage
- Sub retinal hemorrhage
- Intra retinal hemorrhage
Leakage of hard exudates
- Sub RPE/Subretinal/Intraretinal hard exudates
Subretinal hyperreflective material (SHRM)
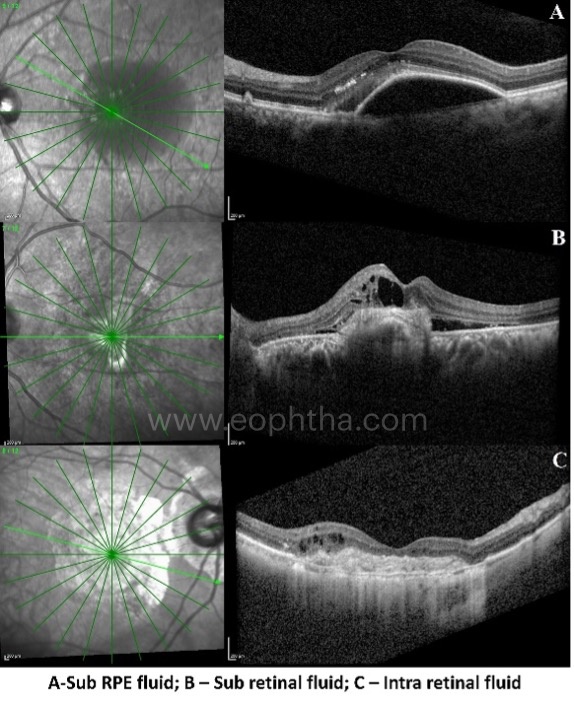
6. Other OCT features which signify CNVM activity:
A. Bacillary Layer Detachment (BALAD):
Bacillary layer detachment (BALAD) is described as a separation of the retinal photoreceptor layer into the myoid inner layer and ellipsoid outer layer on OCT. The mechanism of BALAD in CNVM could be either due to the underlying poor choroidal perfusion which leads to the stress and splitting of the photoreceptor (bacillary) layer or due to a rapid influx of exudative fluid into the potential space between the external limiting membrane and ellipsoid zone as suggested by Jung et al.The photoreceptors receive their nutrients from the choriocapillaris; hence, in the acute stages, the compromised perfusion could lead to a splitting of the bacillary layer. Another possible explanation could be the accumulation of subretinal hyperreflective material (SHRM) or hemorrhage within the bacillary space. The shearing force due to SHRM or hemorrhage causes the separation of the myoid and ellipsoid portions of the photoreceptor layer leading to BALAD. The BALAD resolves once the choroidal neovascular membrane regresses following therapy or sometimes depends on the resolution of the SHRM or hemorrhage within the intrabacillary space.
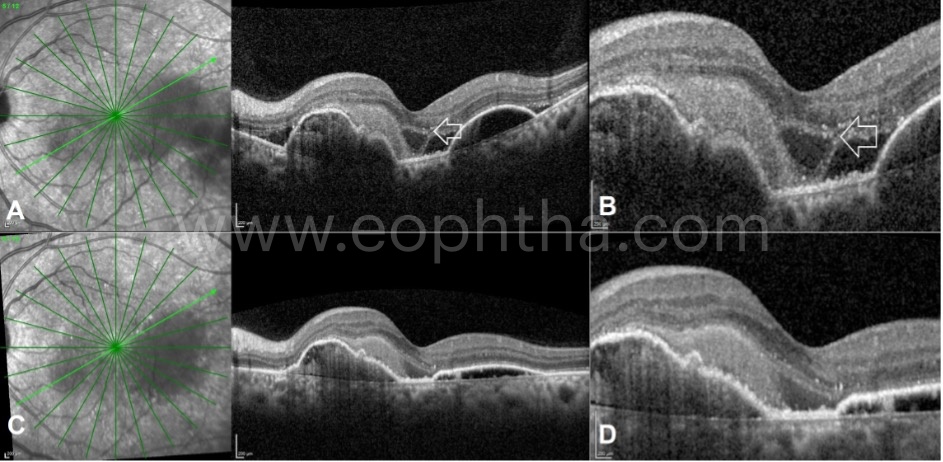
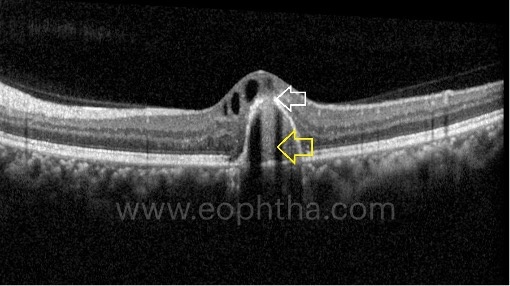
B. Bridge arch-shaped subretinal fluid:
Bridge arch-shaped SRF is an uncommon finding on OCT seen in eyes with subfoveal predominantly scarred variety of CNVM and is characterized by neurosensory detachment in a bow-shaped manner with a steep angle at the junction between the RPE and the sensory retina, and characterized by the presence of adhesion areas between the sensory retina and CNVM complex. Most eyes show type 1 CNVM prior to the development of bridge arch-shaped SRF; but is commonly associated with the type 2 variety at the time of its development. In approximately 70% cases, the SRF responds to intravitreal anti-VEGF injections as early as one month; however, improvement in visual acuity is not seen in these eyes following therapy at the final follow-up visit. Development of large RPE tears is noted in 1/3rd of the CNVM cases with bridge-arch shaped SRF.

C. Hard exudates and cholesterol crystals:
The hard exudates are composed of lipid and proteinaceous material, such as fibrinogen and albumin that leak from the impaired blood–retinal barrier. The hard exudates can get accumulated either in the sub retinal space and/or intraretinal space in patients with exudative neovascular membrane. The Onion Sign was first described by Mukkamala et al as a novel spectral-domain OCT finding of layered hyperreflective lines in the sub-retinal pigment epithelium (RPE) space, usually associated with chronic exudation from type 1 neovascularization (NV) in patients with age-related macular degeneration (AMD).Typically associated with intense signal upon near-infrared reflectance scanning laser ophthalmoscopy (NIR-SLO), the Onion Sign was proposed by its discoverers to represent layers of precipitated lipid amidst chronic exudationafter also considering collagen or fibrin. Others authors suggested fibrovascular scarring,mechanical strain on Bruch’s membrane, and dystrophic calcification in drusen regression as possible histological correlates.
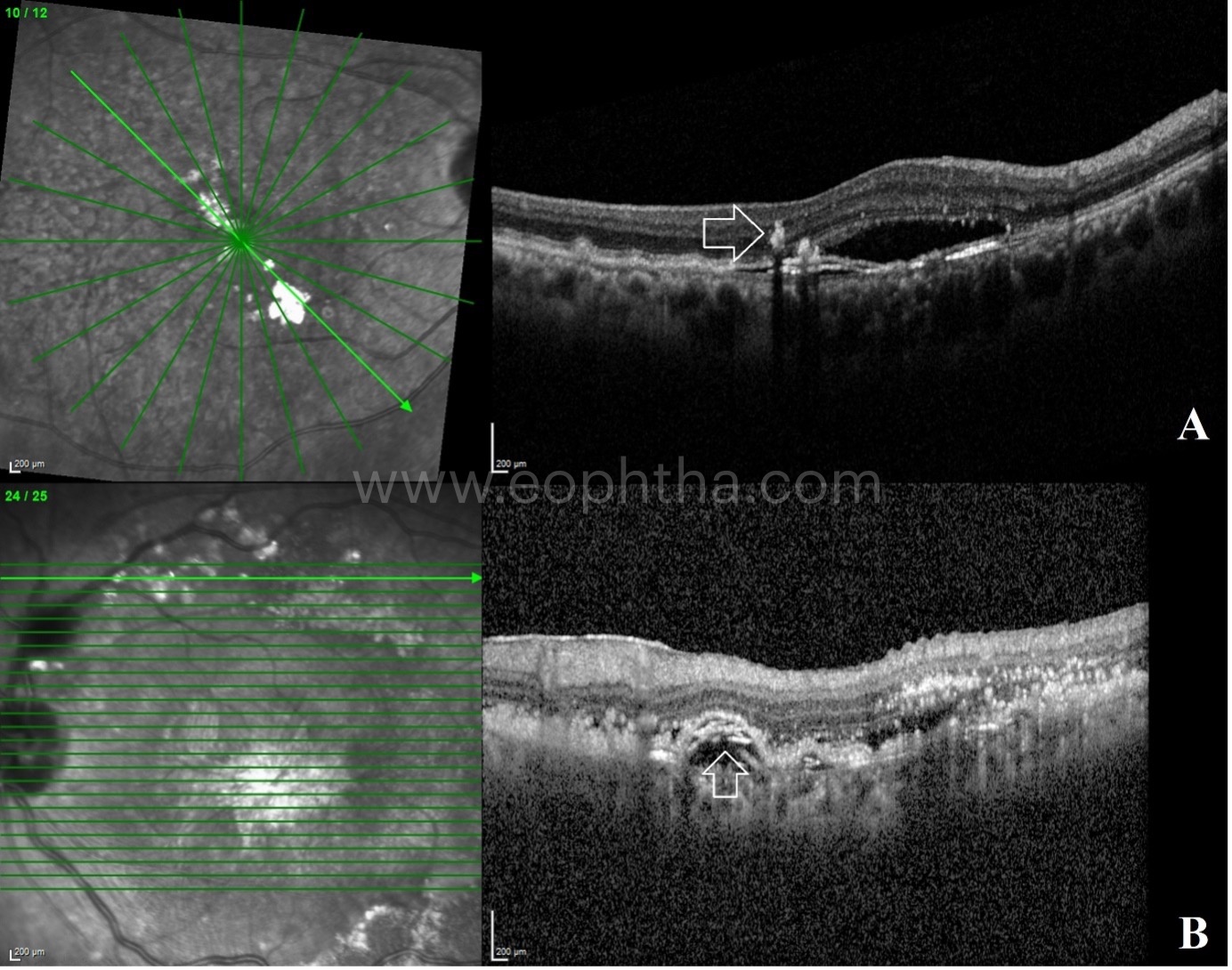
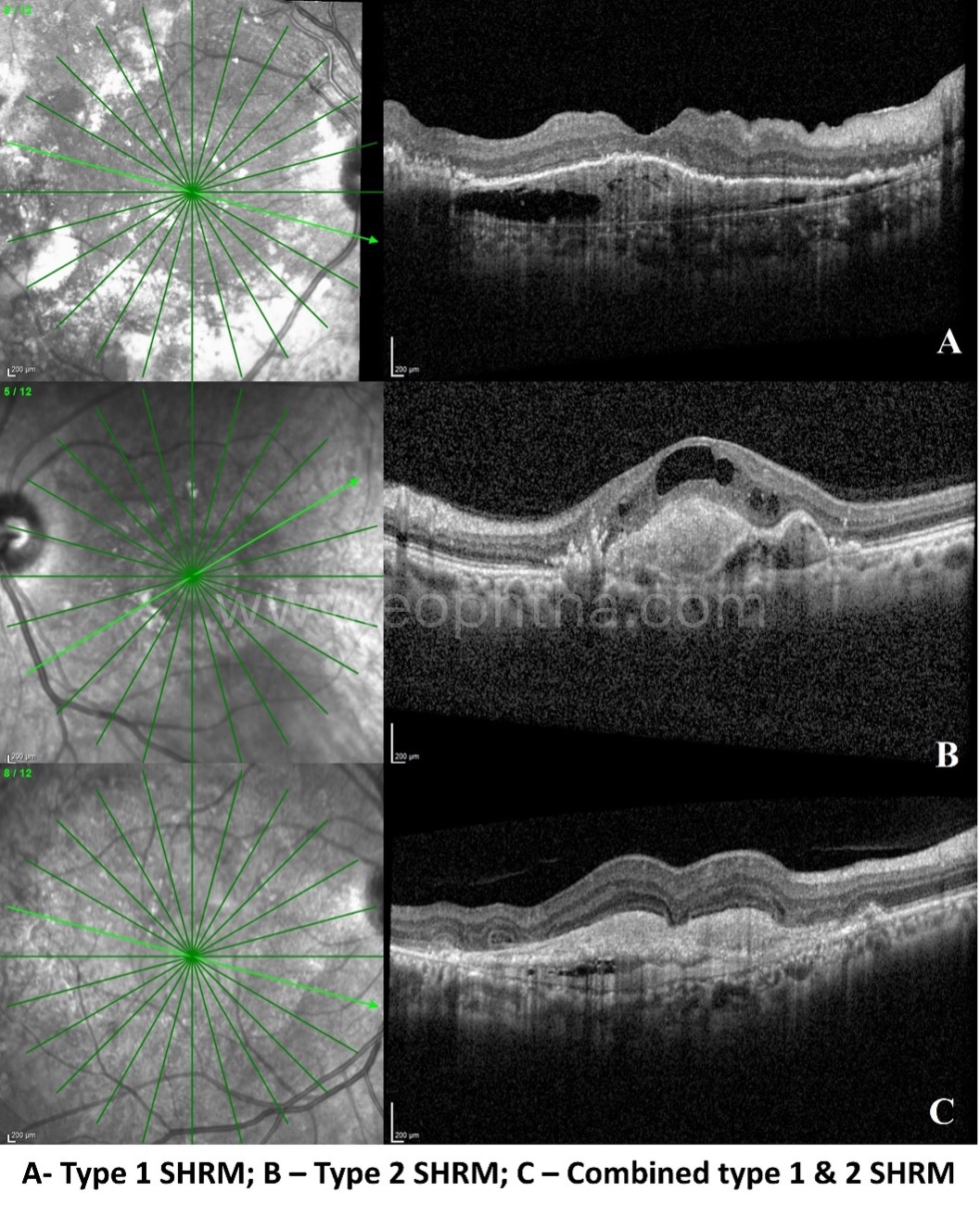
D. SHRM (Subretinal Hyper Reflective Material):
Subretinal hyper-reflective material (SHRM) is a morphological feature seen on optical coherence tomography (OCT) as hyper-reflective material composed of a mixture of heme, fibrin and exudates and located either internal to the RPE (type 1 SHRM) or external to the RPE in the retina (type 2 SHRM).
Clinically relevant features of SHRM:
- Presence of new SHRM indicates disease activity
- With treatment, there is consolidation and resolution of SHRM
- Two types
- Type 1 – Sub RPE SHRM
- Type 2 – Subretinal SHRM
- Active SHRM – has fuzzy borders
- Regressing SHRM – has well-defined borders
SHRM can be considered as a surrogate OCT biomarker in predicting final visual outcome in CNVM. Baseline parameters predicting poorer vision were presence of SHRM at the fovea, well-defined SHRM borders, greater SHRM height, width and area and persistence of SHRM after anti-VEGF therapy.
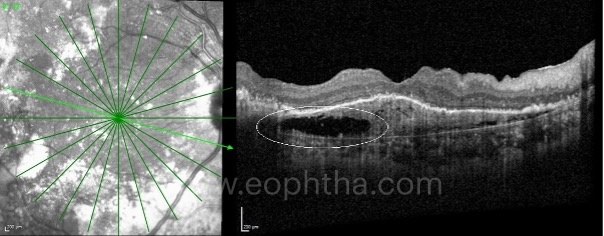
E. Prechoroidal Cleft:
Prechoroidal cleft is characterized by the presence of hyporeflective space between Bruch’s membrane (BM) and hyperreflective materials within PEDs in patients affected by CNVMs. Prechoroidal cleft was found in 15% of Caucasian nAMD patients treated with anti-VEGF injections. Prechoroidal cleft increased in association with CNVM reactivation and decreased after treatment. It represents an accumulation of fluid actively exudating from the MNV and should be considered a sign of neovascular AMD activity. Eyes with prechoroidal cleft required more anti-VEGF injections. The presence of prechoroidal cleft correlates with disease activity, and intensive anti-VEGF suppression can preserve vision.
F. Sub RPE hyperreflective columns:
Salient features include:
Sub-RPE Hyperreflective Columns look like narrow columns of hyperreflectivity beneath the RPE.
They have been considered as a sign of a weakened or cracked RPE layer, where fluid, blood, and/or vessels can more easily break into the subretinal space.
They are described in 27% of eyes with neovascular AMD.
They are different from geographic atrophy and RPE aperture.
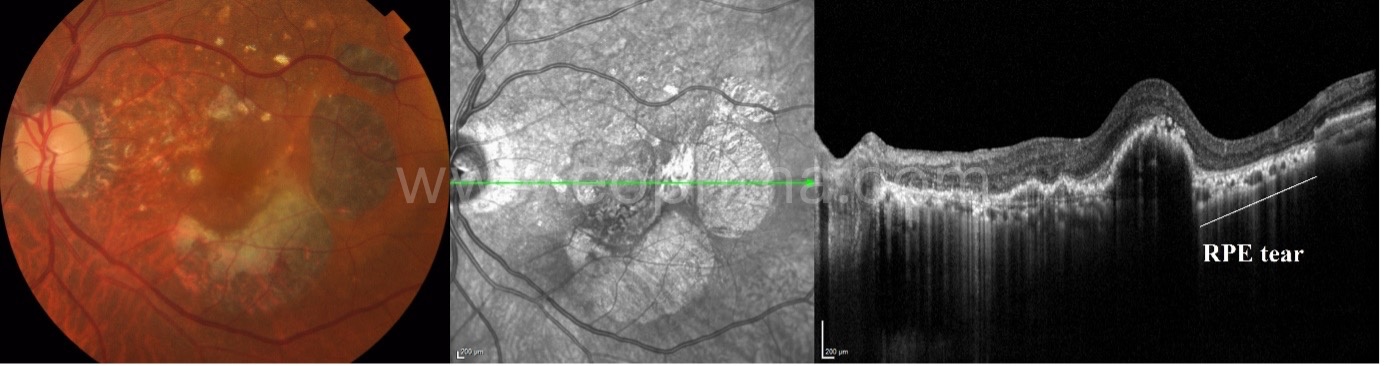
G. RPE tears/ RPE rip:
Retinal pigment epithelial (RPE) tears, also known as RPE rips, represent a disruption of the RPE monolayer. RPE tears are a well-known complication of nAMD. RPE tears occur in patients with pre-existing vascularised PEDs. RPE tears could occur spontaneously or as a consequence of thermal laser treatment, photodynamic therapy, or anti-VEGF therapy. The size and recent onset (<4.5 months) of PED are potential risk factors for developing RPE tears. Different morphologies of PED additionally increase the risk of developing an RPE tear. 80.6% of tears come from a fibrovascular PED, 16.2% after a haemorrhagic PED, and 3.2% from serous PEDs. Sarraf et al. described that a height >550 microns is a high-risk factor for the development of RPE tears. In addition to PED height, a large PED basal diameter is also a risk factor. In addition, a small MNV size/PED size ratio (<50%) has been suggested as a risk factor by Chan et al.
Based on the size of RPE tear, there are 4 grades:
- Grade 1 - <200micron
- Grade 2 – 200-1000micrcon
- Grade 3 - >1000micron
- Grade 4 - >1000 micron and involving the fovea
Grade 4 RPE tear is associated with poor visual outcomes.
7. OCT features which signify CNVM inactivity:
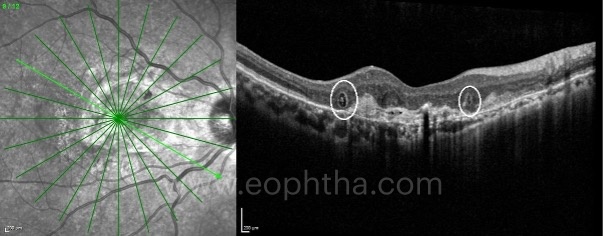
A. Outer Retinal Tubulations (ORTs):
ORTs are identified as hyporeflective structures surrounded by a hyperreflective band on OCT B scans. Their tubular appearance can be better appreciated inen faceOCT images. ORTs can be considered as a rearrangement of photoreceptors as a consequence of retinal injury, and their presence has been associated with worse visual prognosis in neovascular AMD patients. They are not specific for neovascular AMD and are seen in other long standing retinal diseases affecting the photoreceptors as in hereditary macular dystrophies.
B. Disciform scar:
Disciform scars form the stage of exudative AMD in which the retinal tissue is replaced by scar tissue. Disciform scars develop with regression of subretinal hemorrhages and retinal edema and hyperplasic elements of the RPE. Disciform scars are usually dry and show marked destruction of all retinal layers. It is less common to find diffuse exudation with elevation of the entire sensory retina at the posterior pole.

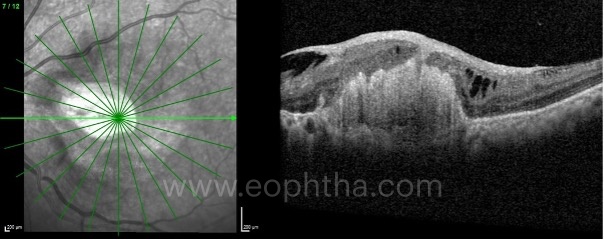
C. Central posterior vitreous fibrosis:
Central Posterior Hyaloidal Fibrosis (CPHF) is a newly reported OCT finding associated with advanced CNV, which may represent a possible profibrotic influence of aCNVMto the overlying posterior hyaloid adhesion. This could be due to an increase in profibrotic mediators in some eyes with advanced CNVM that may cause this unusually adherent posterior hyaloid to thicken at the foveola resulting in “filling in” of foveolar depression.
In conclusion, OCT biomarkers in CNVM are suitable to predict visual acuity, and to guide the treatment and follow-up of these patients, improving the quality of CNVM management.


