One of the major microvascular complications of diabetes mellitus is diabetic retinopathy. Diabetic macular edema (DME) and proliferative diabetic retinopathy are the most common causes of vision loss in diabetic retinopathy.
Pathogenesis of DME:
A simplified understanding of the pathogenesis of DME formation is depicted in Figure 1:
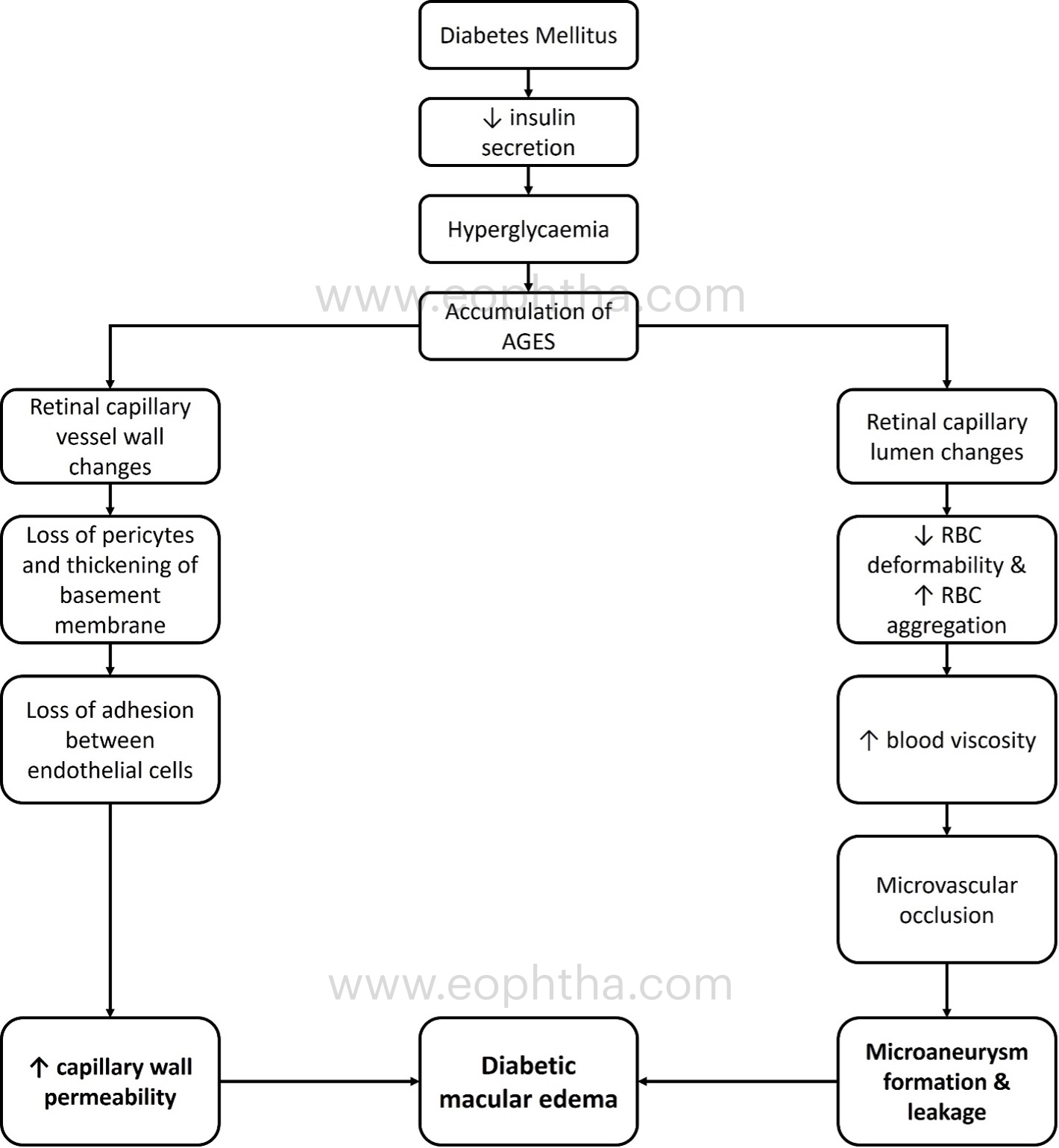
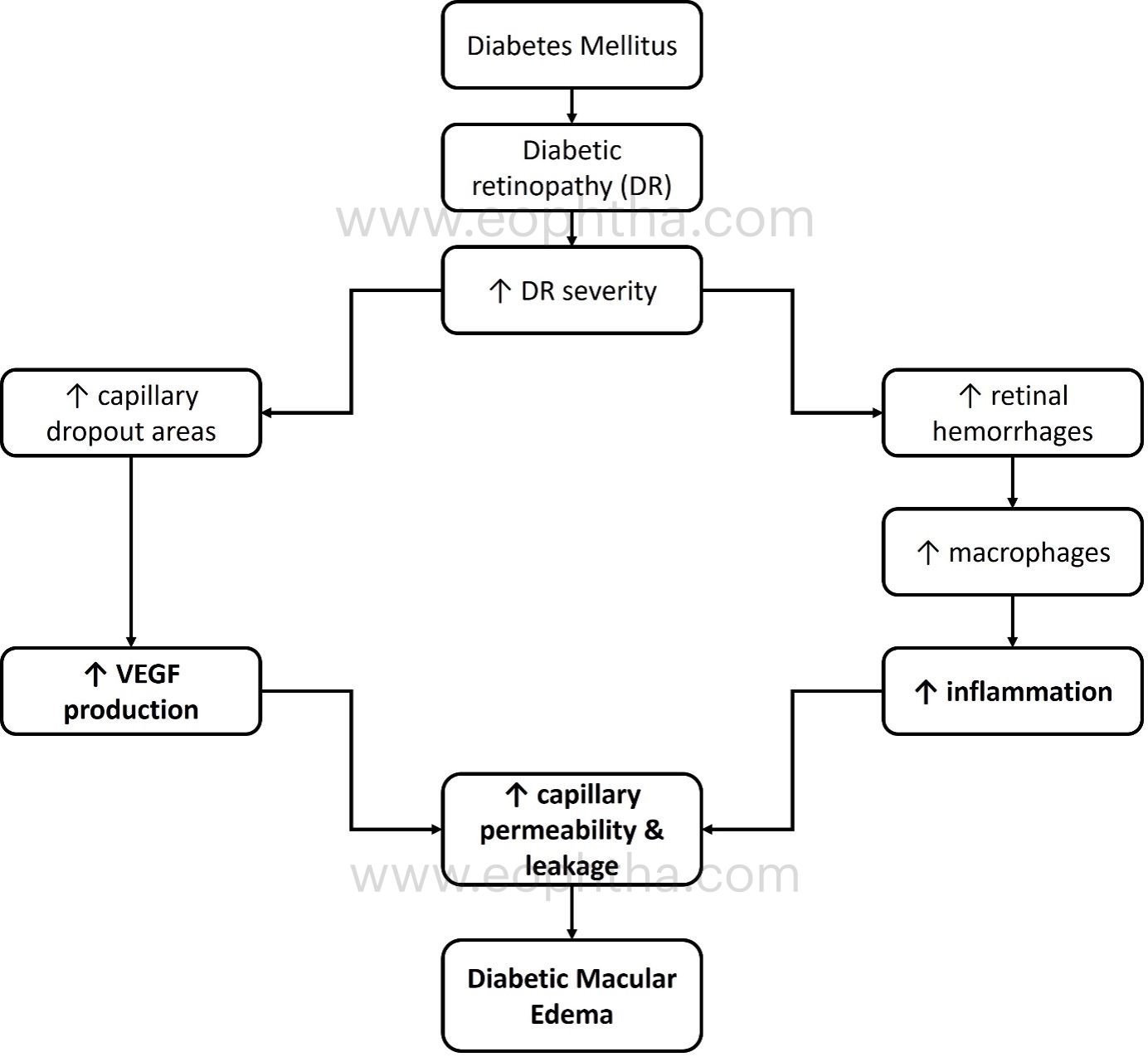
The role of vascular endothelial growth factor (VEGF) and inflammation in the pathogenesis of DME is depicted in Figure 2:
OCT is a non-invasive imaging technique that is widely used in routine retina practice to obtain a high-definition cross section of all retinal layers and to measure their thickness. Recent advances in OCT imaging, such as the introduction of spectral-domain and swept source-based optical coherence tomography (OCT) and enhanced depth imaging, have enabled clinicians to detect and understand the various aspects of DME formation and treatment. This article will discuss the role of various OCT imaging biomarkers in identifying diabetic macular edema and their potential contribution to its management.
OCT in diabetic retinopathy and DME is useful for:
- Identifying the disease at an early stage
- Identifying the type of DME
- Establishing the position of DME
- Recognizing disease manifestations (type of edema)
- To determine the severity of the leak
- Identifying the effects of long-standing macular edema
- Evaluate the function of inflammation in DME
- Identifying macular ischemia
- Correlate with serum biomarkers
- Predict visual prognosis
Steps required while reading an OCT in a case of DR with DME:
A. Identifying the DR changes at early stage:
Formation of microaneurysms is the first clinical sign of diabetic retinopathy. Vertically oval structures with hyperreflective walls and hypo/hyperreflective lumen in the inner nuclear/outer plexiform layer zone suggest involvement of the deep capillary vascular plexus. In addition to DR, they can also be observed in branch retinal vein occlusion, type 1 MacTel, exudative perifoveal vascular anomalous complex (ePVAC), and retinal capillary macroaneurysm. The presence of MA indicates that the retina's microvascular plexus is afflicted.
B. Identifying the type of DME on OCT:
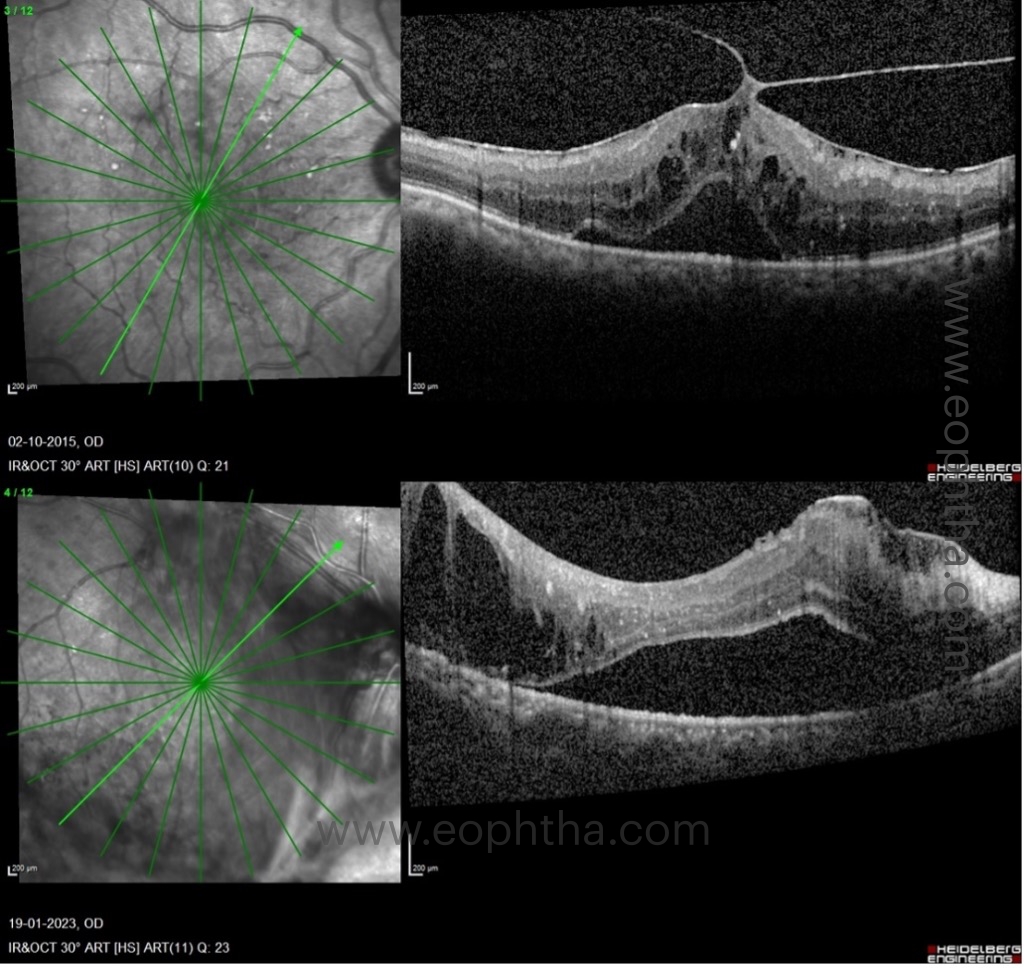
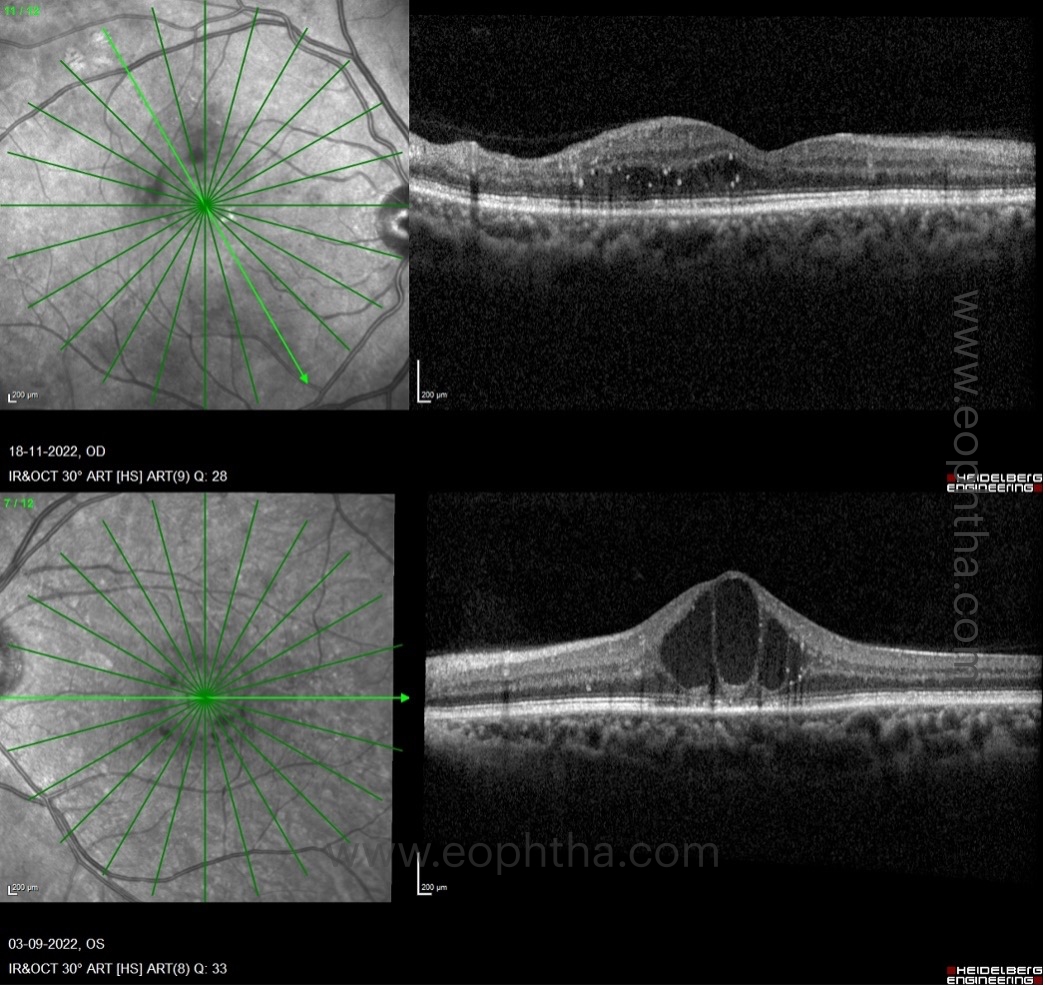
C. Location of DME:
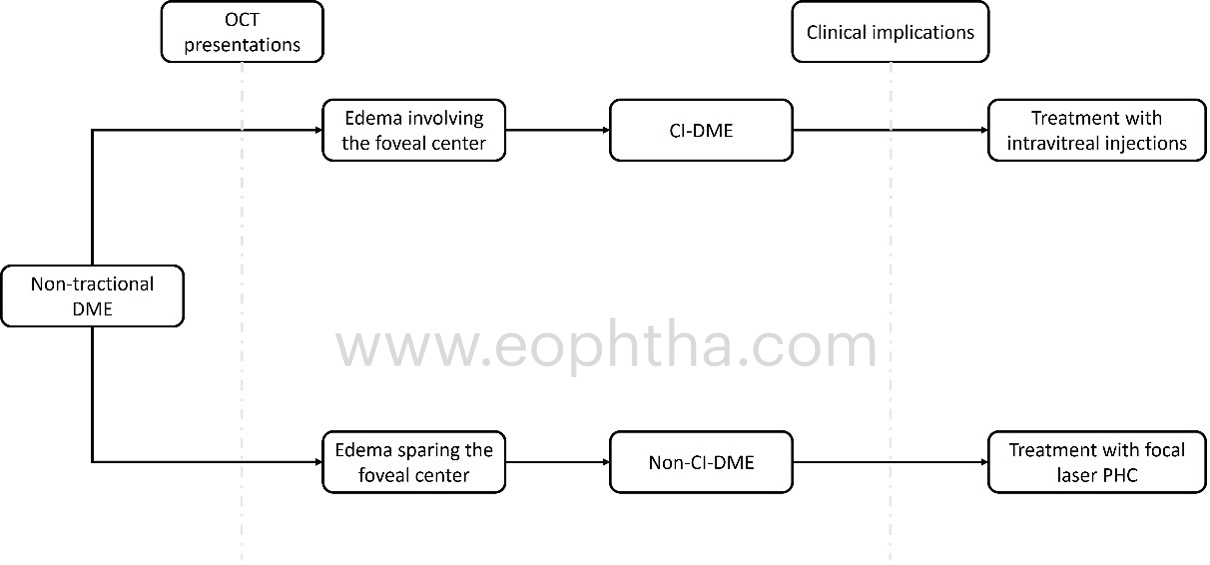
Based on its location, DRCR.net clinical trials classify DME as one of two types: A) DME involving the center and B) DME not involving the center.
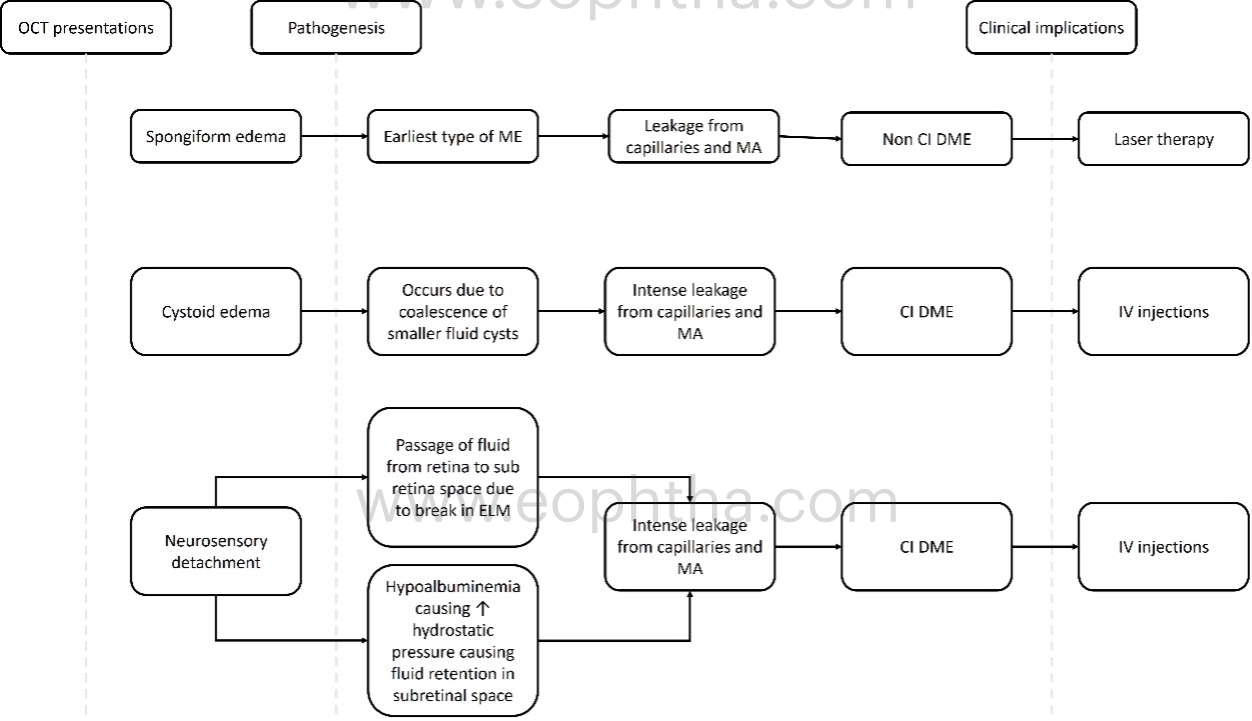
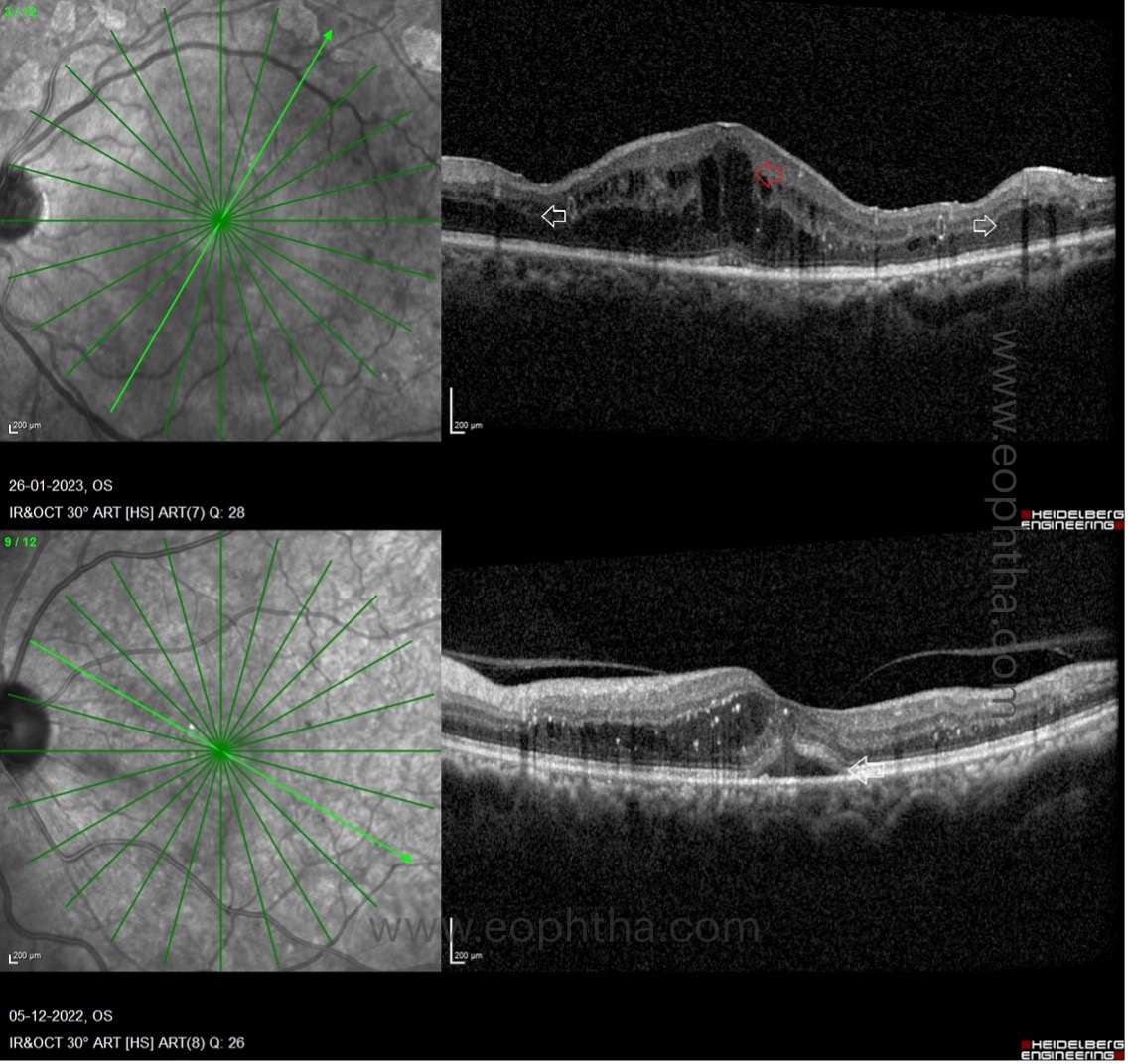
OCT patterns of DME:
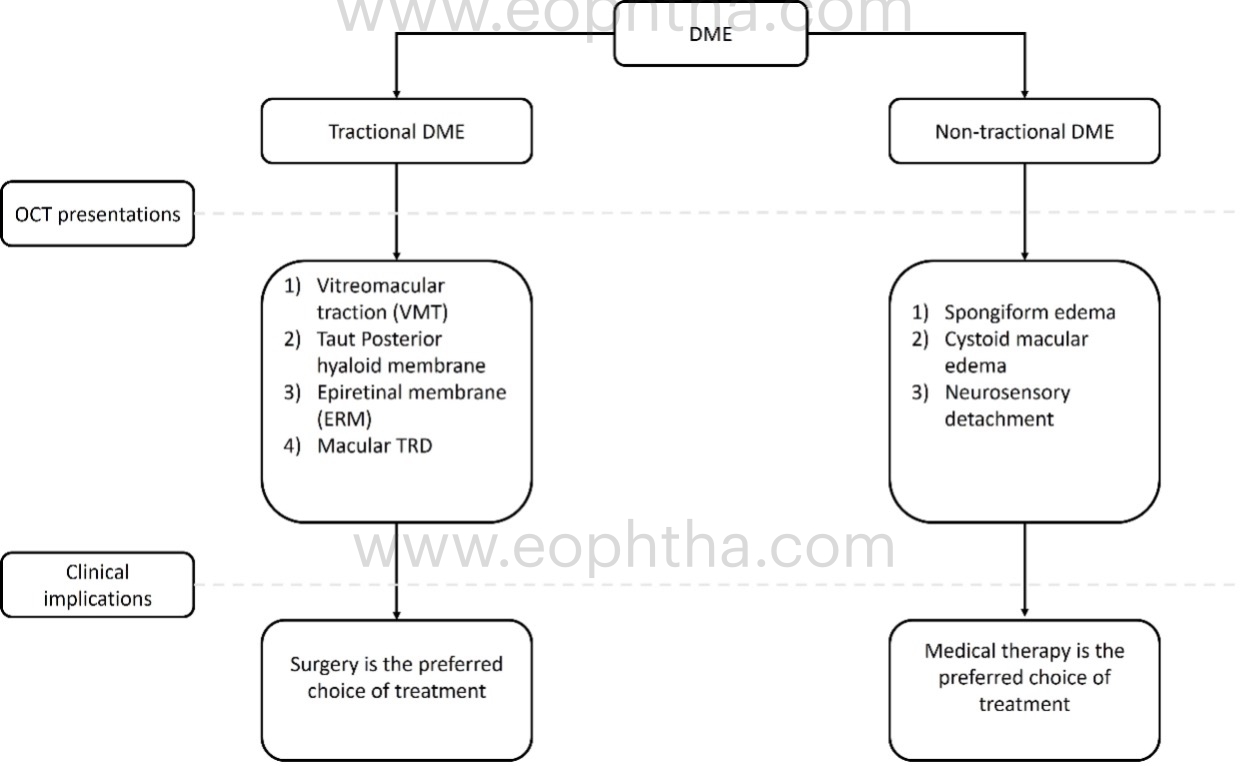
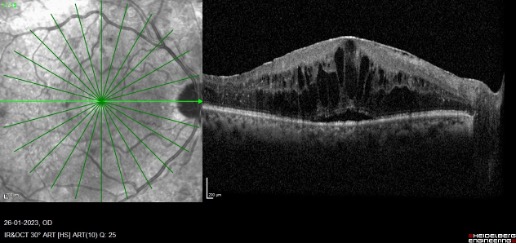
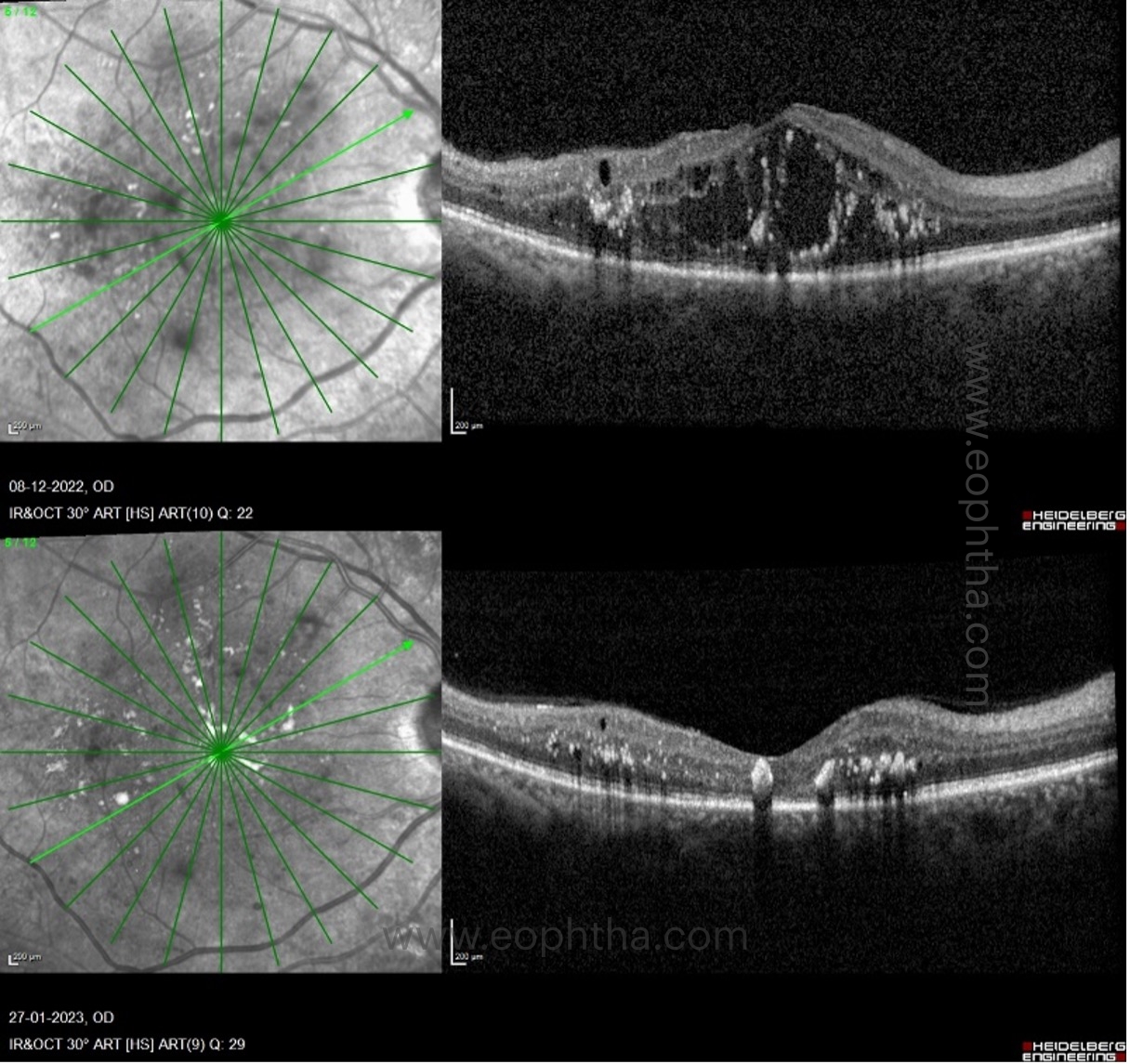
There are three patterns of macular edema: 1) spongiform edema – this is a sponge-like retinal swelling mainly confined to the outer retinal layers due to backscattering from intraretinal fluid accumulation; 2) cystoid macular edema (CME) – these are large fluid-filled cyst-like spaces involving variable depths of the retina with intervening septa mainly confined to the outer retina; and 3) neurosensory detachment (NSD) – a subretinal region of hyporeflectivity. Figure 9 describes the pathogenesis associated with the formation of these patterns and their clinical implications.
D. Recognizing the OCT markers which signify increased severity of leakage:
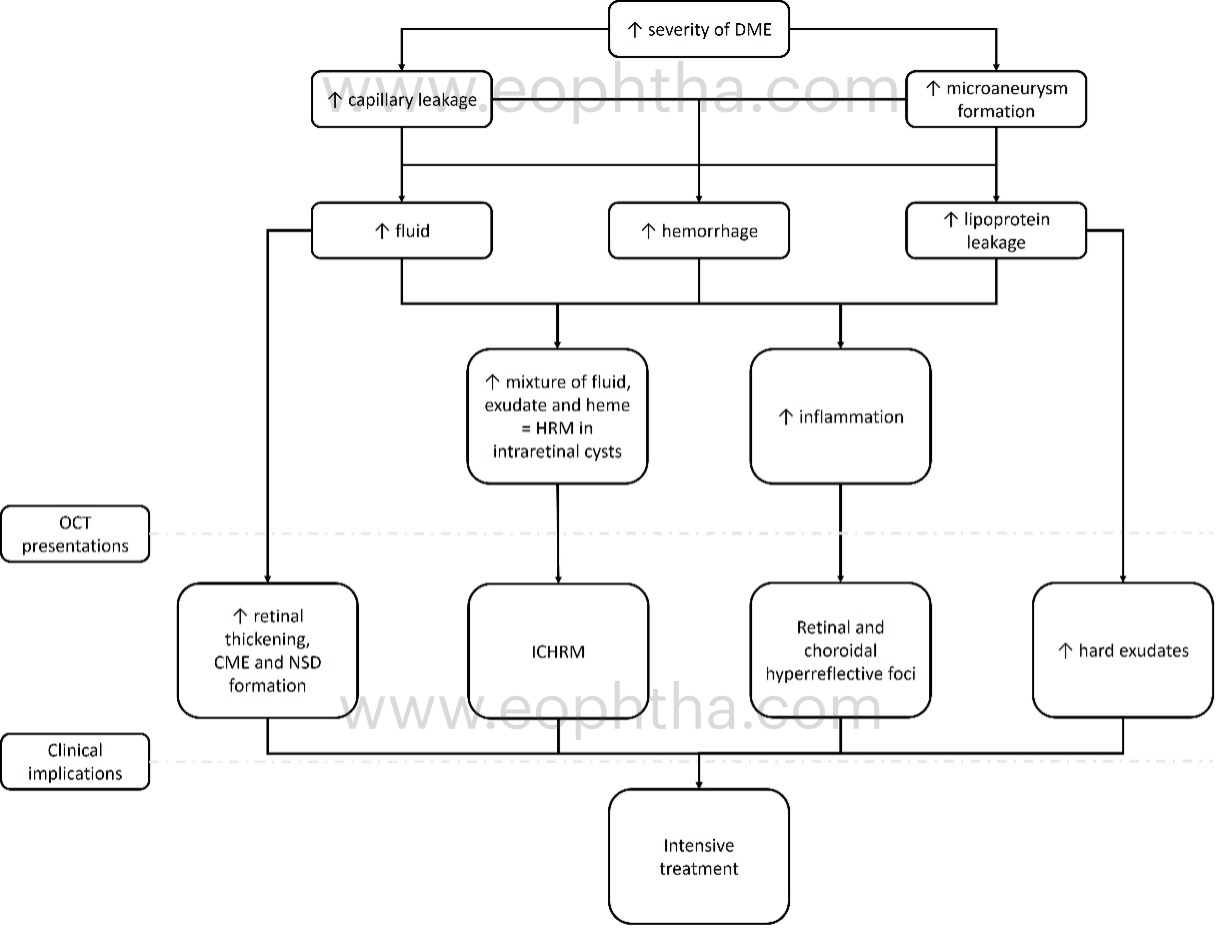
a. Central macular thickness:
Every OCT imaging software includes an ETDRS thickness map that divides the macular region into nine subfields: a 1mm diameter central ring centered on the fovea, and 3mm and 6mm inner and outer macular rings respectively. Central macular thickness is the retinal thickness computed at the central ETDRS circle with a diameter of 1 mm. Increased central macular thickness indicates increased retinal capillaryand/or microaneurysm leakage. Compared to eyes with minimal central retinal thickening, increased CMT indicates intensive therapy, a better response to treatment, a greater reduction in macular edema, and a significant improvement in visual acuity.
b. Cystoid macular edema and neurosensory detachment:
Spongiform edema is the earliest pattern of retinal edema observed in eyes with DME. Spongiform edema is caused by diffuse capillary leakage, predominantly from the deep capillary plexus, resulting in fluid accumulation in the outer retinal layers, namely the outer nuclear layer and outer plexiform layers. With further persistent retinal vascularleakage, the small intraretinal fluid cysts coalesce to form larger cysts with interstitial septa. In addition, an increase in vessel leakage can cause a breach in the external limiting membrane, allowing fluid to transfer from the retinal to subretinal space and cause neurosensory detachment. In addition, diabetes-related hypoalbuminemia increases hydrostatic pressure, resulting in fluid retention in the subretinal space. Consequently, the presence of large cystoid spaces with or without neurosensory detachment indicates increased vessel leakage, necessitating intensive treatment for DME in such cases.
c. Hard exudates:
These are lipo-proteinaceous aggregates found in the outer plexiform layer of the retina as a result of leaky capillaries and microaneurysms. On OCT, they appear as hyperreflective spots larger than 30 microns with back-shadowing. They are more prevalent in individuals with abnormal lipid metabolism. These can either be absorbed by treatment, transform into permanent cholesterol crystals, or migrate centripetally toward the fovea when macular edema is suddenly reduced. For the prevention and treatment of hard exudate formation, intensive intravitreal anti-VEGF or steroid therapy is necessary.
d. Intracystic hyperreflective material (ICHRM):
On OCT, ICHRM is distinguished from intraretinal hyperreflective dots or hard exudates by the presence of clumps of hyperreflective material within the intraretinal cystic space without shadowing. DME is characterized by the accumulation of intraretinal or subretinal fluid, lipid exudates, or both, as a result of microaneurysm leakage and/or hyperpermeability of the vessel walls in the deep capillary plexus. In addition, there is an increase in intraluminal pressure and interstitial pressure distal to the microvascular occlusion, resulting in the transudation of proteins, fibrin, macrophages, fluid, lipids, and/or heme from the intravascular space to the extravascular space. This causes hyperreflective material to accumulate within the cyst. Depending on the area occupied by the ICHRM within the intraretinal cyst, the presence of ICHRM generates variable fluorescent signals. Partial occupancy of the ICHRM by retinal cysts indicates extensive leakage and necessitates additional intensive treatment. Complete occupancy by the ICHRM within the retinal cyst, on the other hand, indicates a longer duration of edema and poor treatment response. Absorption of ICHRM can occur spontaneously or after treatment. In eyes with other retinal or choroidal vascular diseases, such as acute branch retinal vein occlusion and neovascular age-related macular degeneration, similar hyperreflective materialhave been described.
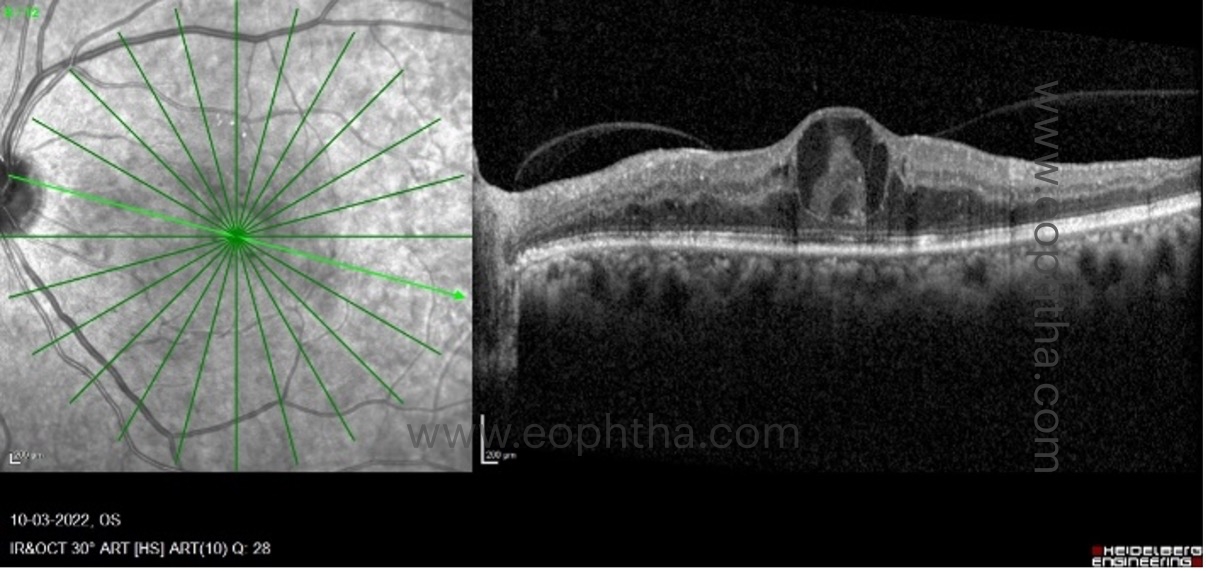
E. OCT biomarkers which help in determining the longer duration of edema:
a. Bridging retinal processes:
Between the cystic cavities, bridging retinal processes are visible as vertically oriented hyperreflective lines. They represent residual neural elements that connect the outer and inner retina, thereby facilitating the transmission of visual impulses from the inner retinal layers to the optic nerve axons. Although the precise composition of these tissues is unknown, it is believed that Muller cells and bipolar cells play a significant role. After treatment, their presence is associated with enhanced visual acuity. In contrast to this, absence of these bridging retinal tissues between inner and outer retina have poor prognosis post treatment. These eyes are unlikely to improve despite resolution of cysts and end up with foveal atrophy and thinning.
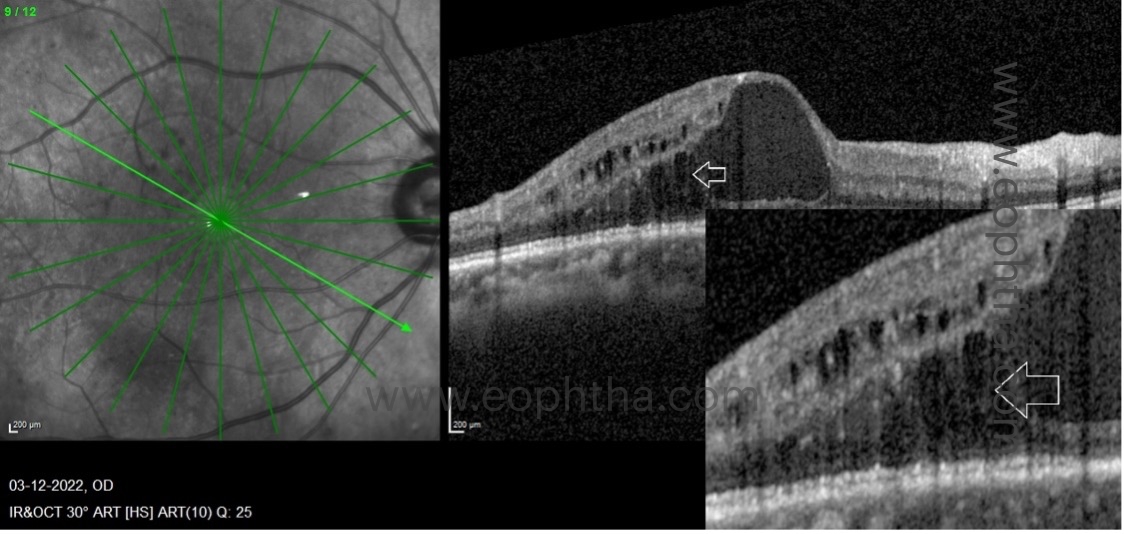
b. Pearl necklace sign:
The pearl necklace sign on OCT refers to hyperreflective dots arranged in a contiguous ring around the inner wall of cystoid spaces in the retina, in eyes with chronic exudative diabetic maculopathy.There is uniform homogenous turbidity within the cyst suggestive of the chronic nature of the edema.
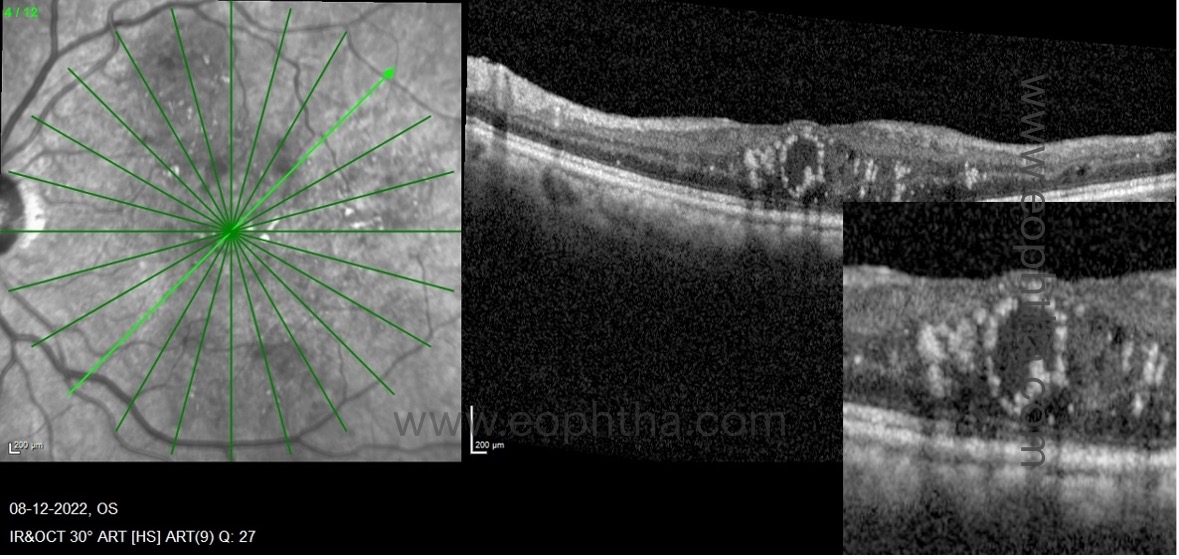
F. OCT imaging markers which suggest inflammation in DME:
a. Hyperreflective retinal foci:
HRF appears as intraretinal hyperreflective dots on OCT in patients with retinal pathologies such as DME. They are not unique to DME and can also be observed in patients with retinal vascular diseases, such as retinal vein occlusions, and choroidal diseases, such as age-related macular degeneration. They are subclinical, meaning they are not visible during a fundoscopy. Some clinicians believe they are subclinical lipoprotein deposits that have accumulated as a result of the breakdown of the blood retinal barrier. The widely accepted theory is that the hyperreflective retinal foci are activated microglial cells, indicating that the disease process is inflammatory. Others believe they are degenerated photoreceptors that are migrating towards the inner retina. They must be distinguished from hard exudates. In the following table, the distinctions between hyperreflective retinal foci and hard exudates are outlined:
|
Hyperreflective retinal foci |
Hard exudates |
|
|
Size |
<30microns |
>30microns |
|
Location |
Inner retinal layers |
Outer retinal layers |
|
Shadowing |
No back shadowing |
Back shadowing present |
|
Reflectivity |
Reflectivity similar to RNFL |
Reflectivity similar to RPE-Bruch’s complex |
Important imaging markers of retinal inflammation are HRF. After treatment with anti-VEGF and corticosteroid implants, the size and number of HRF may be reduced. In a recent study, it was found that corticosteroid implants had better outcomes than anti-VEGF agents in patients with DME and HRF. The study revealed that a higher initial HRF count responded better to dexamethasone implants than anti-VEGF therapy. Recent research indicates that a greater number of HRF on OCT is associated with early recurrence of DME following steroid implant. As a result, patients with a high HRF count on OCT should be closely monitored for early and intensive treatment, if necessary.
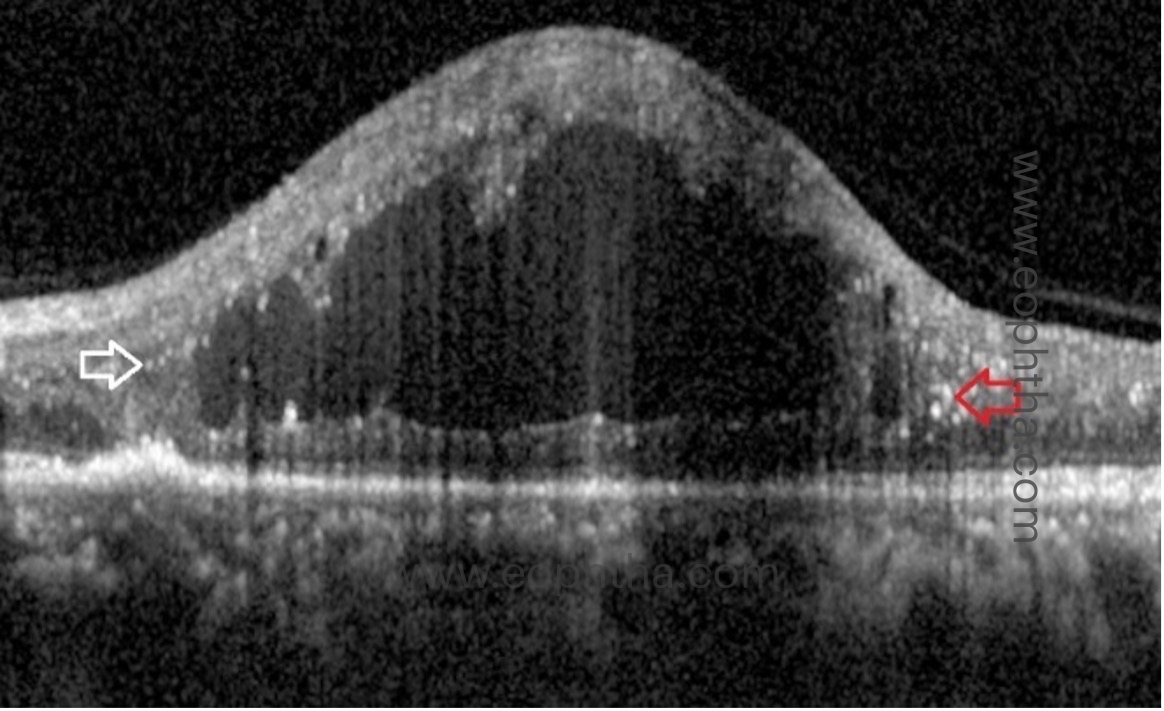
G. Identifying macular ischemia on OCT:
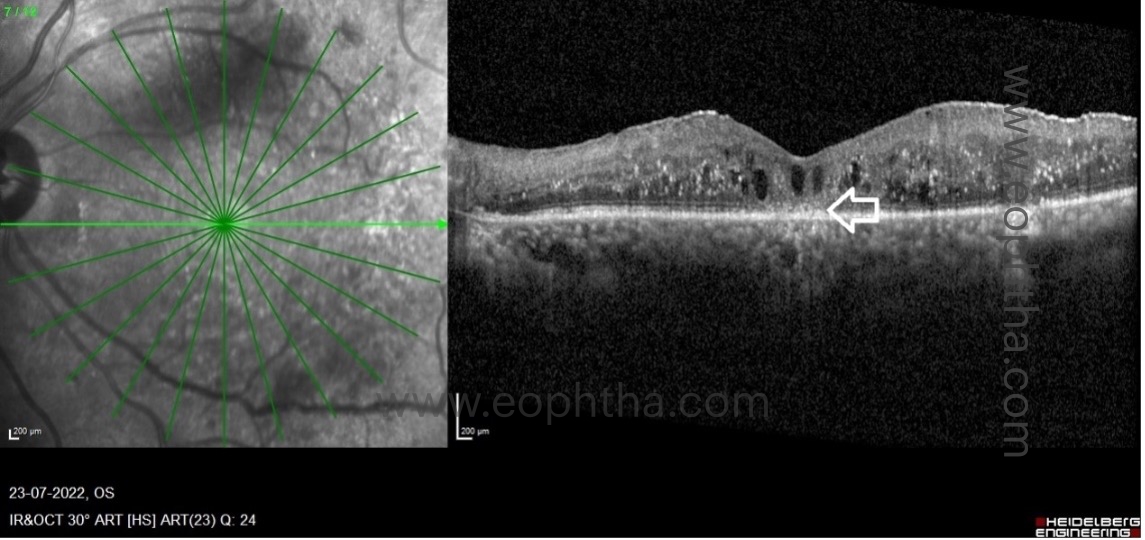
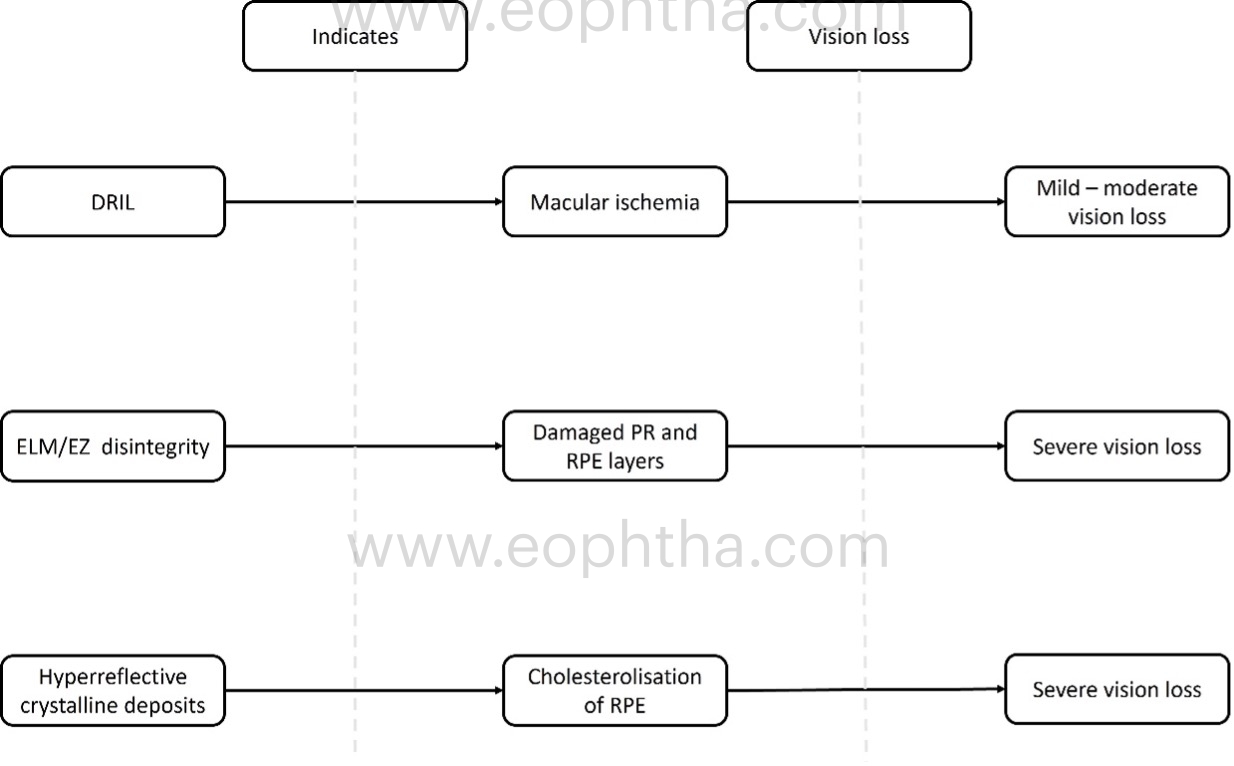
a. Disorganisation of retinal inner layers (DRIL):
DRIL is the inability to differentiate between the ganglion cell layer–inner plexiform layer complex, inner nuclear layer, and outer plexiform layer. The inner retinal layers are composed of axons, bipolar cells, and amacrine cell nuclei, all of which are crucial for the transmission of visual signals from photoreceptors to the ganglion cell layer. The vessels of the deep capillary plexus supply these layers. The loss of these capillaries damages these structures, resulting in abnormal visual processing and the formation of DRIL. Therefore, the presence of DRIL on OCT correlates with capillary flow void regions on OCT angiography and macular ischemia on FFA. DRIL has been identified as a reliable biomarker for visual acuity prognosis in DME. On OCT B-scans, DRIL is measured by examining the central 1 mm of the retina. Disorganization of more than 50% or more than 500 micrometres of this area is considered significant and is associated with a poorer visual prognosis in eyes with edema or resolved edema. A rise in DRIL over four months predicted a one-line decline in visual acuity. The presence of DRIL causes mild to moderate vision impairment, but not severe impairment.
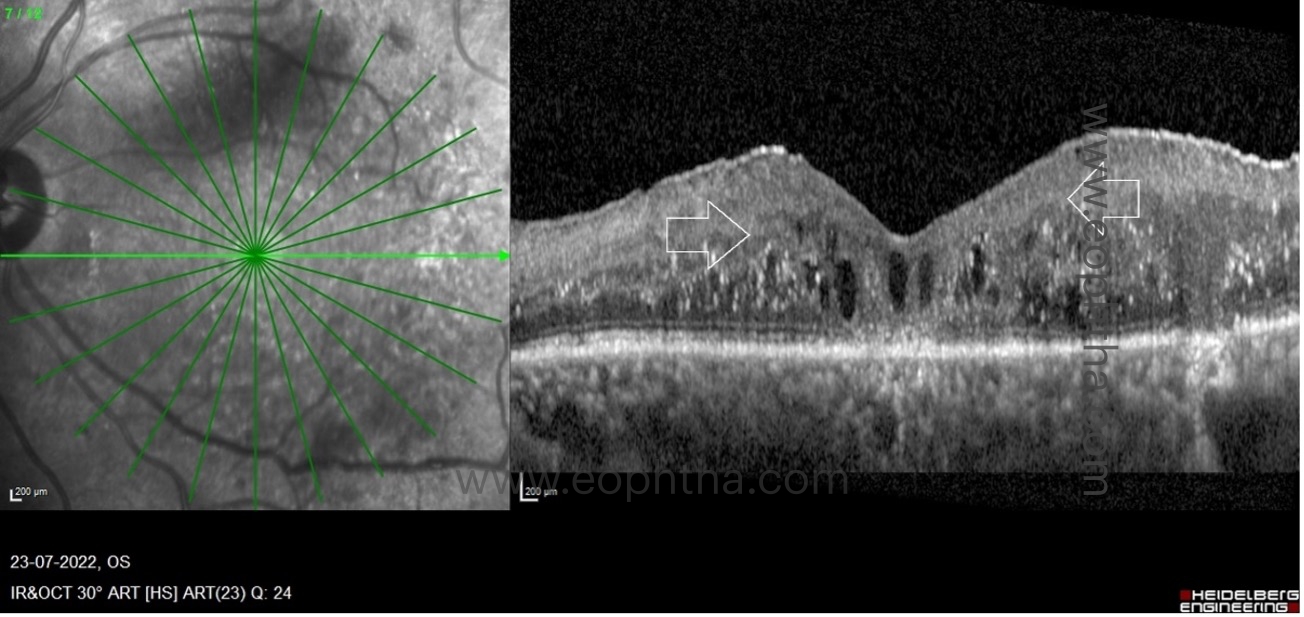
H. Imaging markers correlating with serological markers:
Cholesterol crystals (CC) or hyperreflective crystalline deposits (HCD):
On spectral domain OCT, HCDs or CCs appear as single or multiple, linear, hyperreflective lines of high intensity on or above the RPE without posterior shadowing. HCD is observed in or above the RPE in cases of DME, i.e., on the RPE, in the subretinal space above the RPE, and in the neurosensory retina above or below the OPL. On ophthalmoscopy and color fundus photography, HCDs are identified as brilliantly yellow-white or golden-yellow lesions. On Multicolour® imaging, lesions can be seen as highly reflective lesions on the individual color reflectance channels based on their location in the retina. These lesions are distinct from the commonly observed HEs, which appear as yellow flecks in the retinal arcades of DR patients. Plaques of hard exudate/lipid exudate are typically observed in a circinate pattern peripheral to the area of leakage or as large, confluent exudations. With OCT, HEs exhibit posterior shadowing and can be detected at the outer plexiform layer, subretinal space, or both. The horizontal and planar, single or multi-layered HCDs detected by OCT in DME represent intraretinal or subretinal cholesterol crystal deposits identical to the "onion ring sign" observed in neovascular AMD. HCDs or CCs in diabetic maculopathy indicate chronic vascular leakage, hypercholesterolemia requiring lipid-lowering medications, and involvement of the fovea can result in significant visual impairment.
Differences between hyperreflective crystalline deposits and hard exudates:
|
Hyperreflective crystalline deposits |
Hard exudates |
|
|
Appearance of clinical examination |
Shining golden-yellow or yellow white lesions |
Dull yellow flecks |
|
Orientation in OCT scans |
Horizontally oriented |
Irregular clumps |
|
Location in the retina |
RPE, Subretinal space and intraretinal space |
Subretinal space and intraretinal space |
|
Shadowing on OCT |
Absent |
Present |
|
Visual prognosis |
Permanent vision loss if the RPE at the fovea gets involved |
Poor vision till the hard exudates remains at the fovea. Vision improves once the hard exudates resolve. |
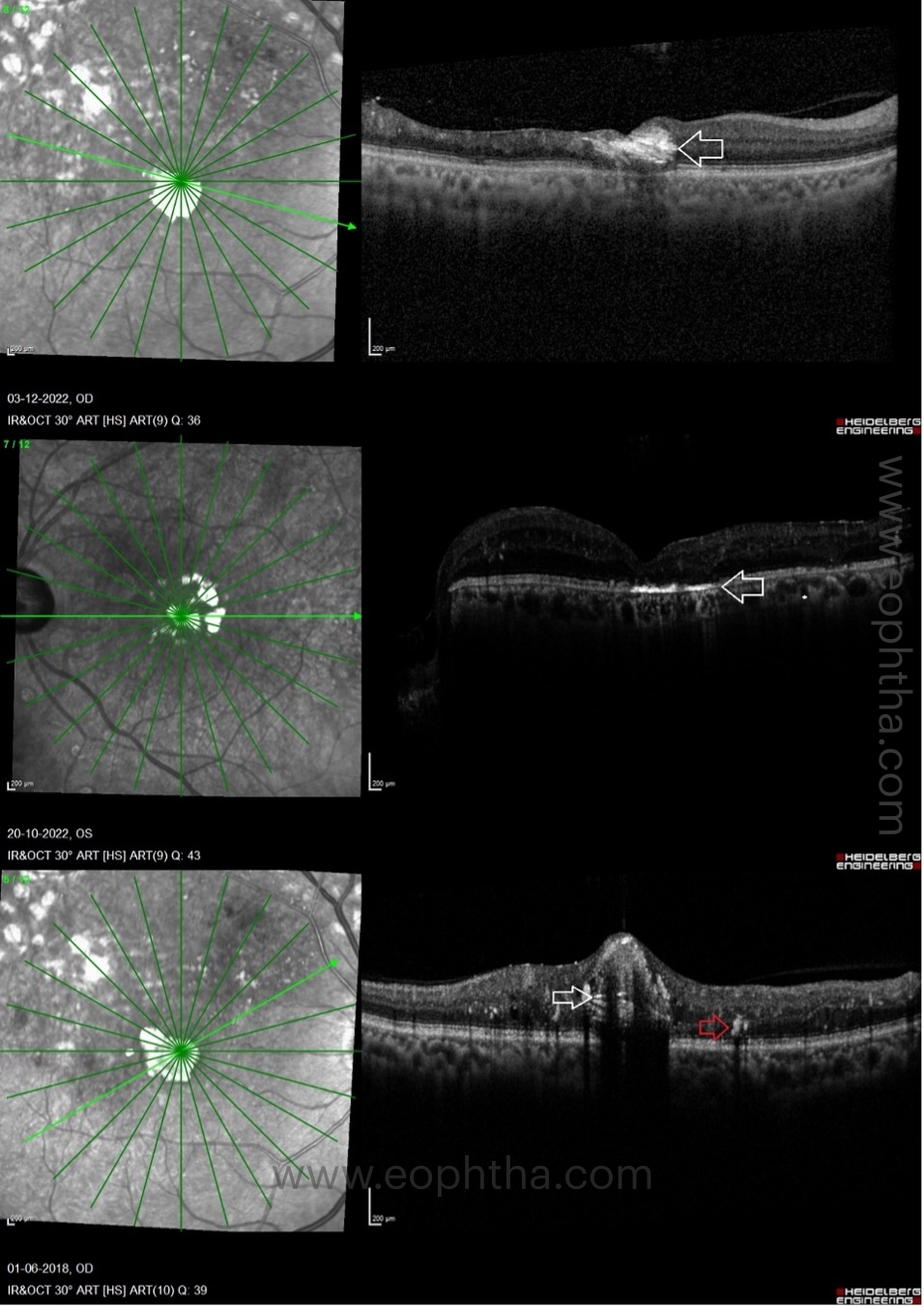
Factors which contribute to poor visual prognosis:
- DRIL – already described above
- Cholesterol crystals or hyperreflective crystalline deposits
- ELM (external limiting membrane) /EZ (ellipsoid zone) discontinuity:
The integrity of the outer retinal layers is a direct indicator of photoreceptor and RPE health. Studies indicate that eyes with an intact EZ junction experience greater visual gains following treatment. There are three levels of EZ continuity: completely continuous, partially disrupted, and completely disrupted. Subjects with long-standing DME may demonstrate focal or diffuse loss of ELM and EZ. Visual acuity gains in patients with outer retinal damage have been found to be suboptimal, according to research. Visual acuity has a positive correlation with the ELM and EZ survival rate.
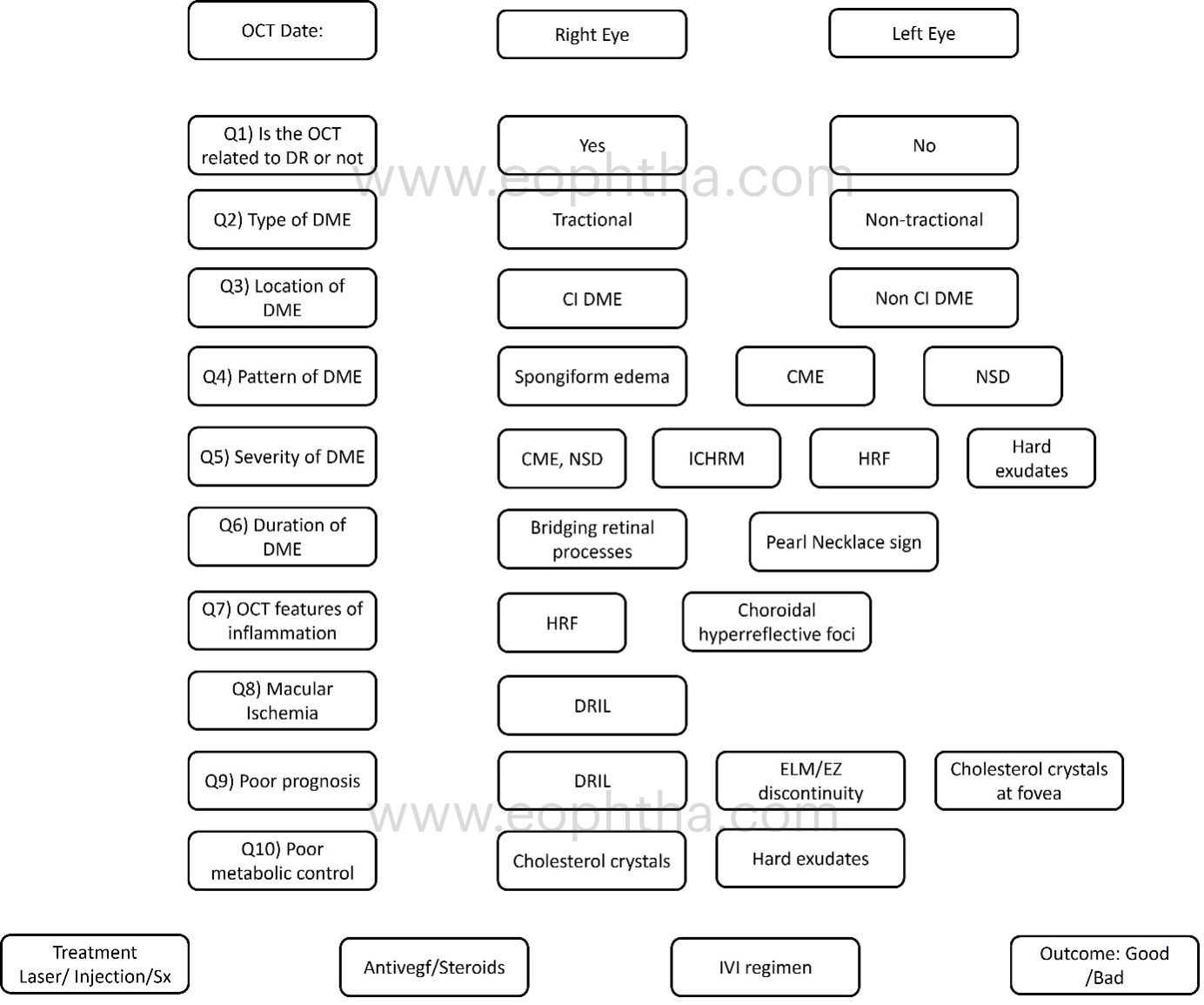
This worksheet would assist the clinician in noting all pertinent findings in a patient with DME, planning the appropriate treatment, and anticipating the best outcome.
With the advent of SD and SS OCT, retinal and choroid microstructural details can be readily perceived. This imaging method aids in the detection of subclinical disease, even in hazy environments and without the complications associated with the invasive dye-based imaging modality.


