Ocular involvement in TB has been recognized for a long time and was first described in 17111. The spectrum of tuberculosis (TB)-related uveitis is wide. Its diagnosis still remains a challenge due to the lack of uniformity in the diagnostic criteria along with difficulties encountered in confirming the diagnosis by the laboratory methods available. It is a major concern in countries endemic for TB, and intraocular TB is being increasingly reported from various regions2-4.
The true prevalence of intraocular TB is not known in India. While it has been reported to be 0.39% in South India, it is much higher (9.86%) in North India1,5. Varying rates of its prevalence have been reported from the world over (0.5% in the U.S.A., 6.31% in Italy, 6.9% in Japan, and 10.5% in Saudi Arabia). The largest series of patients with presumed intraocular TB and their outcome has been recently reported from our center6.
Pathogenesis
Uveitis due to latent TB has been reported since long. However, their exact association is not known. Intraocular TB commonly occurs in the absence of concurrent pulmonary TB, as any other form of extrapulmonary TB6,7. The primary infection in any form of TB is acquired through lungs via the inhaled droplets. The alveolar macrophages serve as the primary hosts and release various cytokines and chemokines. The monocytes get transformed into epitheloid cells and giant cells, forming the epitheloid cell granuloma with central area of necrosis containing the acid-fast bacilli (AFB). During the initial infection, the AFB-containing monocytes enter into the circulation, reach the extrapulmonary organ(s) and remain dormant. Later, it is the reactivation of these dormant monocytes at some stage of life that causes active TB infection. It is believed that about 10% of patients with latent TB will go on to develop active TB at a later stage in their life 8.
Clinical features
Intraocular TB has a wide clinical spectrum that may include anterior uveitis (granulomatous /chronic uveitis), intermediate uveitis, posterior uveitis including retinal vasculitis, serpiginouslike choroiditis, choroidal tubercles or granuloma, subretinal abscess, or panuveitis1. In our clinical experience, broad-based posterior synechiae (figure 1), retinal vasculitis with or without perivascular choroiditis and serpiginouslike choroiditis are significant clinical predictors of tubercular etiology of uveitis in the presence of latent TB9. The rare manifestations include endophthalmitis, panophthalmitis and neuroretinitis10-12. Immunocompromised individuals may have atypical presentations like hypopyon uveitis, or endophthalmitis.
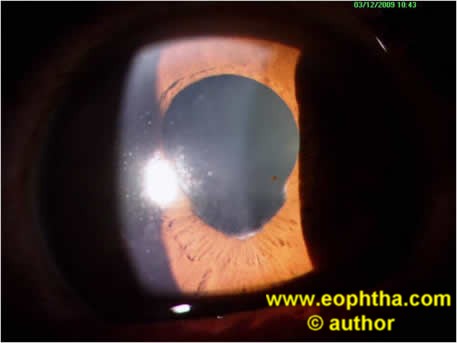
Figure 1. Broad-based posterior synechiae in a patient with presumed tubercular chronic anterior uveitis.
Chronic granulomatous anterior uveitisis commonly seen as mutton fat keratic precipitates, iris nodules, and posterior synechiae. Cataract and extensive broad-based posterior synechiae are formed as a result of chronic inflammation. Snowballs and pars plana exudates (snowbank) along with peripheral vascular sheathing and cystoid macular edema are features of intermediate uveitis. Sometimes, only a spillover of inflammatory cells from the anterior chamber may produce mild vitritis. The posterior segment is the most common site of involvement in intraocular TB with typical features that help to differentiate from nontubercular causes of posterior uveitis13.
Retinal Vasculitisdue to TB has been shown in several studies. Retinal veins are more commonly affected than the arteries. In our experience, patients with retinal vasculitis who had a positive polymerase chain reaction (PCR) forMycobacterium tuberculosisfrom the intraocular fluid exhibited certain characteristic features14. These include moderate vitritis, perivascular cuffing with infiltrates, retinal hemorrhages, peripheral capillary nonperfusion causing neovascularization of the optic disc or elsewhere in the retina, and neuroretinitis (figure 2). The presence of choroiditis lesions (active or healed) under the retinal vessels indicates a strong possibility of tubercular etiology 9.
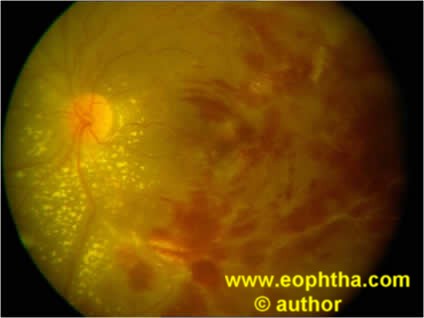
Figure 2. Tubercular Retinal vasculitis shows perivascular infiltrates with superficial hemorrhages and peripapillary exudates .
Tubercular serpiginouslike choroiditis(SLC) frequently affects young to middle-aged males. It may present in three different morphological forms15. The most common presentation is the appearance of multifocal lesions of choroiditis in the posterior pole and periphery that expand centrifugally and later coalesce in a serpiginoid pattern. The juxtapapillary region is usually spared. The active lesions are yellowish-white clinically with raised edges and fuzzy margins that appear hypo fluorescent in the early phase of FFA and hyper fluorescent in the late phase (figure 3). It may also manifest as a single, large, placoid lesion with active edges that appear raised. The centre of the lesion shows variable degree of pigmentation as the lesion heals centripetally. Occasionally, one eye may have multifocal lesions and the other eye may have placoid lesion, causing a mixed pattern. Classic serpiginous choroiditis (SC) can be clinically differentiated from tubercular SLC by involvement of the juxtapapillary region. A positive uveitis workup for TB, frequent presence of vitritis and preferential involvement of the posterior pole and periphery further characterize tubercular SLC.16
Choroidal tubercles/Granuloma:The most characteristic clinical presentation is the choroidal tubercles that represent granulomas located deep in the choroid. They present as grayish-white to yellowish lesions, discrete with indistinct borders and typically elevated in the centre. They show early hypofluorescence and late hyperfluorescence on fundus fluorescein angiography (FFA). These are usually few in number and most commonly found in the posterior pole. Mononuclear infilterates and granuloma with caseation necrosis that may contain acid-fast bacilli are the histological findings suggestive of a hematogenous spread ofMycobacterium tuberculosisfrom primary site of infection. Once healed, they produce scars with pale, atrophic, sharply demarcated borders and variable pigmentation. Rapid multiplication of bacilli leads to a progressive, liquefied caseation necrosis and tissue destruction causing formation of a subretinal abscess. It appears as a yellowish, solitary, elevated subretinal mass-like lesion, frequently associated with surrounding exudative retinal detachment (figure 4)17.
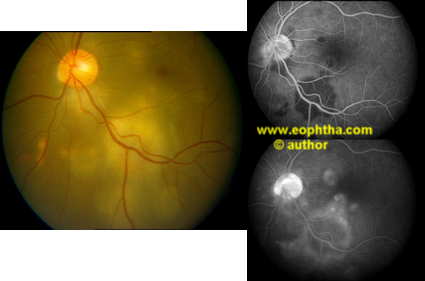
Figure 3. Left eye of patient shows serpiginouslike choroiditis in the inferotemporal quadrant with raised active lesions that appear hypofluorescent in the early and hyperfluorescent in the late phases of fundus fluorescein angiography.
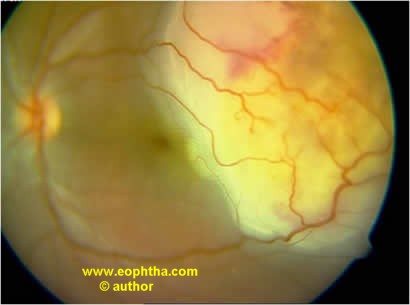
Figure 4. A large solitary choroidal tubercular granuloma mimicking a subretinal abscess in the upper temporal quadrant.
Diagnosis
The gold standard for diagnosing intraocular TB is the demonstration ofMycobacterium tuberculosisfrom the intraocular fluid or tissues by microbiological or histopathological examination. The currently available laboratory methods provide an indirect evidence of tubercular etiology of uveitis. The diagnosis of intraocular TB still remains largely presumptive as the ocular tissue is rarely sampled. The use of specific clinical signs as markers predicting TB as the cause of uveitis in a TB-endemic area has been recently reported by us9. Definitive diagnosis of extraocular TB in a patient with uveitis also aids in diagnosing intraocular TB.
Indirect evidence
The century-old tuberculin skin test TST has been widely used to diagnose latent TB infection. Latent TB is diagnosed when a person is infected withMycobacterium tuberculosisbut does not have active tuberculosis disease. Following an intradermal injection of 0.1 ml of 5 tuberculin units of purified protein derivative in the forearm, the amount of induration (and not erythema) is measured after 48-72 hours. A person who has been exposed to the bacteria is expected to mount an immune response in the skin containing the bacterial protein. A positive test (induration>10 mm) indicates latent TB. This indurated response represents the type IV immune response characterizing delayed-type hypersensitivity. In our population, a positive TST along with presence of any of the clinical signs such as broad-based posterior synechiae, retinal vasculitis (with or without choroiditis) or SLC in a patient with no other detectable cause of uveitis is highly suggestive of intraocular TB9. The TST is, however, limited by two-step process, non-reproducibility, booster effect, low sensitivity and specificity. Some of these limitations are overcome by the introduction of interferon-γrelease assays (IGRAs). These include the QuantiFERON-TB Gold In-Tube (QFT) (Cellestis Inc., Carnegie, Australia) and ELISpotPLUS (T-SPOT.TB, Oxford Immunotec, Abingdon, U.K.) 18. The QFT is approved for use in the United States (Food and Drug Administration, 2007) and is being widely used in many countries. It measures and compares the IFN-γ response of T cells toMycobacterium tuberculosisantigen. It is an objective, single-visit blood test and more specific than the TST 19. It is less affected by BCG vaccination. However, it has not been found superior to TST in sensitivity for use as a screening test or first-line study in TB-related uveitis. Moreover, it is technically difficult and expensive.
The T-SPOT.TBtest is approved in Europe and is under evaluation by the FDA. It is based on overnight enzyme-linked immunospot (ELISpot) assay. It enumerates individual T cells producing IFN-γ after antigenic stimulation. This test is more sensitive than TST for diagnosing LTBI. In the various studies done so far, the T-SPOT.TBtest has been found to have a correlation with the level of exposure to MTB more strongly than the TST. These second-generation tests may hold promise for use in the uveitis clinic
Definitive evidence
Polymerase chain reaction (PCR) is a molecular technique for the evaluation of very small amounts of DNA and RNA by enzymatic amplification of nucleic acid sequences. It offers a significant advantage over traditional methods for identifyingMycobacterium tuberculosisin very tiny samples of body fluids and tissue specimens. It can detect DNA in intraocular fluids where it is present in very minute quantities. There are several reports of PCR-based diagnosis of intraocular TB20-24. More recently, real-time PCR or quantitative PCR has been used in confirming the diagnosis of intraocular TB25,26. It allows fast detection and quantification of pathogen load in the tested specimen, with a minimized risk of carryover and cross-contamination.
Demonstration of acid-fast bacilli on direct smear or culture ofMycobacterium tuberculosisfrom ocular fluids or tissue specimens is laborious and may not be widely available. A number of studies have reported microbiologic and histopathologic evidence ofMycobacterium tuberculosisin ocular specimens. However, a major surgical intervention may be required to obtain the specimen.
Cytokines are important determinants of cellular and molecular activities in TB. Release of proinflammatory cytokines a few weeks after initiating antitubercular therapy causes paradoxical worsening of clinical lesions that is self-limiting27.
Treatment
The dramatic response to ATT in our experience justifies empirical treatment based on a high index of clinical suspicion and positive Mantoux test9. The treatment for ocular TB comprises of the same 4-drug regimen as for any other extraocular form of TB (Isoniazid 5 mg/kg /day, Rifampicin 10 mg/kg/day, Ethambutol 15 mg/kg/day, and Pyrazinamide 20-25 mg/kg/day). The ATT kills the microorganisms and decreases the antigen load. This, in turn, minimizes the inflammation and reduces hypersensitivity reaction, which eliminates recurrence of inflammation. Depending upon the severity of inflammation, the conventional treatment includes corticosteroids (topical and/or oral), initially to control the inflammation, and then tapered and stopped over 6-12 weeks. Immunosuppressive agents may be added as and when required. The exact duration of treatment is not known and ranges between 12 to 18 months. The regimen is revised after a period of 3-4 months, with Ethambutol and Pyrazinamide being stopped. The ATT should always be administered in consultation with the internist specialized in the field of tuberculosis and other systemic inflammatory disorders associated with uveitis. The problems related to ATT are many, such as drug-resistant TB, gastric discomfort, poor compliance, drug toxicity impairing the liver functioning, etc., which need to be dealt with caution.
Various newer agents like rifabutin, fluoroquinolones, interferon-alpha, and linezolid are being tried for drug-resistant TB. The World Health Organization estimates that up to 50 million persons worldwide may be infected with drug-resistant strains of TB. The fatality rate of MDR-TB is 20-80%. At present, 27 potential anti-TB drugs are at various stages of development. The eradication of dormant populations of MTB organisms that cause relapse, using new classes of anti-TB drugs is very promising for the prevention of TB incidence because it will markedly reduce the incidence of active TB from persons who are latently infected with MTB.
REFERENCES:
- Helm CJ, Holland GN. Ocular tuberculosis. Surv Ophthalmol 1993;38:229 –256.
- Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in North India. Indian J Ophthalmol 2004;52:121–125.
- Wakabayashi T, Morimura Y, Miyamoto Y, Okada AA. Changing patterns of intraocular inflammatory disease in Japan. Ocul Immunol Inflamm 2003;11:277–286.
- Islam SM, Tabbara KF. Causes of uveitis at the eye center in Saudi Arabia: a retrospective review. Ophthalmic Epidemiol 2002;9:239 –249.
- Biswas J, Narain S, Das D, Ganesh SK. Pattern of uveitis in a referral uveitis clinic in India. Int Ophthalmol 1996-97;20:223-228.
- Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of antitubercular therapy in uveitis with latent/manifest tuberculsosis. Am J Ophthalmol 2008;146:772-779.
- Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited:a review of experience at Boston City and other hospitals. Medicine (Baltimore) 1984;63:25–55.
- Sutherland I. Recent studies in the epidemiology of tuberculosis, based on the risk of being infected with tubercle bacilli. Adv Tuberc Res 1976;19:1– 63.
- Gupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis.Am J Ophthalmol 2010;149:562-570.
- Rathinam SR, Rao NA. Tuberculous intraocular infection presenting with pigmented hypopyon: a clinicopathological case report. British J Ophthalmol 2004;88:721-722.
- Raina UK, Tuli D, Arora R, Mehta DK, Taneja M. Tuberculous endophthalmitis simulating retinoblastoma. Am J Ophthalmol 2000;130:843-845.
- Stechschulte SU, kim RY, Cunningham ET Jr. Tuberculous Neuroretinitis. J Neuroopthalmol 1999;19:201-204.
- Gupta A, Gupta V. Tubercular posterior uveitis. Int Ophthalmol Clin 2005;Spring;45:71-88.
- Gupta A, Gupta V, Arora S, Dogra MR, Bambery P. PCR-Positive tubercular retinal vasculitis: Clinical characteristics and management. Retina 2001;21:435-444.
- Gupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Ophthalmology 2003;110:1744-1749. Vasconcelos-Santos DV, Rao K, Davies JB, Sohn EH, Rao NA. Serpiginouslike choroiditis in contrast to Classic serpiginous choroiditis. Arch Ophthalmol 2010;128:853-858.
- Gupta V, Gupta A, Sachdeva N, Arora S, Bambery P. Successful mamangement of tubercular subretinal granulomas. Ocul Immunol Imflamm 2006;14:35-40.
- Mazurek GH, Jereb J, Lobue P, et al. Guidelines for using the QuantiFERON-TB Gold Test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005;54:49 –55.
- Kurup SK, Buggage RR, Clarke GL, ursea R, Lim WK, Nussenblatt RB. Gamma interferon assay as an alternative to PPD skin testing in selected patients with granulomatous intraocular inflammatory disease. Can J Ophthalmol 2006;41:737-740.
- Biswas J, ThereseL, Madhawan HN. Use ofpolymerase chain reaction in detection of Mycobacterium tuberculosis complex DNA from vitreous sample of eales’ disease. Br J Ophthalmol 1999;83:994.
- Madhawan HN, Therese KL, Gunisha P, et al. polymerase chain reaction for detection of Mycobacterium tuberculosisin epiretinal membrane in Eales’ disease. Invest Ophthalmol Vis sci 2000;41:822-825.
- Arora S, Gupta V, Gupta A, et al. Diagnostic efficacy of polymerase chain reaction in granulomatous uveitis. Tuber Lung Dis 1999;79:229-233.
- Bowyer JD, Gormley PD, Seth R, et al. Choroidal tuberculosis diagnosed by polymerase chain reaction. A clinicopathologic case report. Ophthalmology 1999;16:290-294.
- Ortega-Larrocea G, Bobadilla-del-Valle M, Ponce-de-Leon A, et al. Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreus of patients with uveitis.
- Rao NA, Saraswathy S, Smith RE. Tubercular Uveitis: Distribution ofMycobacterium tuberculosisin the Retinal Pigment Epithelium. Arch Ophthalmol 2006;124:1777-9.
- Sharma P, Bansal R, Gupta V, Gupta A. Diagnosis of tubercular uveitis by quantitative polymerase chain reaction. J Ophthal Inflamm Infec 2011;1:23-27
- Rathinam SR, Lalitha P. Paradoxical worsening of ocular tuberculosis in HIV patients after antiretroviral therapy. Eye 2007;21:667-668.

