Introduction
Central serous chorioretinopathy (CSC) is one of several chorioretinal disorders characterized by serous detachment of the neurosensory retina and ⁄ or the retinal pigment epithelium (RPE). CSC is one of the 10 most common diseases of the posterior segment of the eye and a frequent cause of mild to moderate visual impairment.1
History of the disease2,3,4,5,6
1866 – Von graefe – first described the disease as recurrent serous retinitis.
1916 – Fuch’s work on the disease was appreciated.
1927 – Horniker – named disease as “Central Angiospastic Retinitis”
1930 – Walsh & Sloane – “idiopathic flat detachment of macula”
1930 – Gifford and Marquardt - Theory on Angioneurotic diathesis.
1953 – Klien – theory on autonomic nervous system dysfunction.
1950’s – Bennett & Maumenee – spectrum of macular disciform degeneration.
1955 – Bennett – “central serous retinopathy”
1960’s – Maumenee and Gass – FA appearance of CSC.
1967 – Gass – “central serous choroidopathy”
Definition
Active CSC is characterized by detachment of the neurosensory retina caused by accumulation of serous ?uid between the photoreceptor outer segments and the RPE in combination with monofocal or multifocal changes in the RPE
Pathogenesis
The patho-physiology of CSC is still not completely understood. It is important to be aware of the anatomy of the choriocapillaris-Bruch's membrane-RPE layer. The widely fenestrated endothelium of the choriocapillaris allows leakage of small protein molecules and fluid into the intercellular space. But the RPE represents an impermeable barrier to the diffusion of fluid into the subretinal space. The RPE pump acts in a vitreous choriocapillaries direction to keep the subretinal space dry. It is difficult to accept that a single, isolated disturbance of a few RPE cells may overwhelm the RPE pump of the neighboring normal RPE. It is plausible to assume that at the basis of the disease there is a more diffuse dysfunction of the RPE cells, the choroid, or both.
RPE dysfunction theory7,8
When evaluating possible patho-physiologic processes at the RPE level, one needs to consider five points:
- The intact RPE creates a barrier between the neurosensory retina and choroid.
- In areas of chorioretinal scar tissue, as occurs after inflammation or photocoagulation, the pigment epithelial diffusion barrier remains permanently destroyed.
- Choroidal capillaries exert a suction on the surrounding fluid.
- The intact RPE absorbs fluid in a retinochoroidal direction.
- Under certain condition, the function of the RPE is reversed, so it secretes in a chorioretinal direction.
RPE damaged via immunologic infections circulatory and neuronal mechanism
↓
RPE secretes ions in chorioretinal direction (towards retina)
↓
Choroidal fluid gets attracted into this area.
↓
Strong flow disrupts the diffusion barrier in this area.
Since the defective area is so small (in the RPE), only a tiny leakage point is visible during the earliest phase of FA. Subsequently, there is rapid increase in fluorescein stained liquid in the subretinal blister during the following stages of angiography. This demonstrates the high speed and large amount of the fluid passing through the diseased area of the RPE.
Choroid dysfunction theory9,10,11
Psychogenic, pregnancy, transplantation, type A, raised cortisol levels
↓
Adrenergic reaction causes damage to the choriocapillaries
↓
Hyperpermeability of choriocapillaries
↓
RPE cell degeneration
↓
Secondary changes in RPE causes leaks
↓
Serous retinal detachment
The hydrostatic pressure of the fluid pooling under the detached RPE will then mechanically cause a solution of continuity in the RPE layer with the subsequent leakage of fluid in the sub retinal space and neurosensory detachment of retina.
Three reports from France have described elevated prevalence of Helicobacter pylori infection in patients with CSC compared to the background population (Mauget-Faysse et al. 2002; Ahnoux-Zabsonre et al. 2004; Cotticelli et al. 2006).12,13,14
Recent evidence suggests that the outer segment of photoreceptors (OS) may elongate and the ONL may become thinner close to a serous retinal detachment. Because the OS is enveloped by RPE microvilli that enable mutual metabolism, the ?rst retinal insult resulting from a RD may be in the photoreceptor OS, which ensures apoptosis of the photoreceptor cell bodies. In its physiologic cycle, the outer part of photoreceptor OS is phagocytized continuously by RPE cell, whereas its inner part is regenerated at the junction with IS. If the neurosensory retina is detached from the RPE, the tip of OS fails to be phagocytized by the RPE, which may develop elongation of the OS. Chronic disturbed renewal of the OS may lead apoptosis of cone cells and thinning of the ONL.
Types:
Clinically, it is of 2 types :-
- Typical or Classic CSC– seen in younger patients & causes an acute localized detachment of retina with mild to moderate loss of visual acuity associated with one or few focal leaks seen during FFA.
- Atypical CSC –Further of 2 types: -
- Chronic CSC or Diffuse retinal pigment epitheliopathy– widespread alteration of pigmentation of the RPE related to the chronic presence of shallow subretinal fluid.
- bullous retinal detachments usually located inferiorly.
Histologically, (Spitznas) it is of 3 types: -
- NSR Detachment.
- RPE Detachment.
- Both NSR & RPE Detachment.
Demography15,16
Age– It affects young to middle aged individuals 20 – 45 years of age. In women age tends to be higher. If age is>50 years– diagnosis is seriously questioned as mostly later it turns out to be “age related macular degeneration” and “choroidal neovascularization”.
Sex– Male predominance – 8 to 10:1
Race–Commonly affects Whites, Hispanics, Asians – Japanese mostly. African-Americans are affected very less. Severe form occurs with - south east Asian and Latin origins.
Systemic associations of the disease17,18,19,20,21,22
- Migraine like headache
- Type A personality
- Hypochondrial behavior
- Hysteria
- Conversional neurosis
- Increased Cortisol levels in patients with Cushing’s disease.
- Long term corticosteroid treatment in organ transplants & Respiratory allergies.
Although the role played by corticosteroids in CSC is not well understood, it is probable that among other mechanisms, the anti- inflammatory properties of steroids may cause delayed healing of the RPE defect. Cortisol, by suppressing synthesis of extracellular matrix components and inhibiting fibroblastic activity, also may damage directly the RPE cells or their tight junctions and may delay any reparative process in damaged RPE cells.
Symptoms
- Small PEDs may be present in macular or para-macular area before the onset of symptoms.
- This is followed by detachment of the neurosensory retina in the surrounding area. If detachment not involving the central macula. Patient remainsasymptomatic &detachment resolves spontaneously.
- If Neurosensory detachment involves the fovea -Symptomatic.
- Metamorphopsia.
- Micropsia.
- Chromatopsia.
- Central scotoma (relative).
- Loss of contrast sensitivity.
- Hyperopia – corresponding to anterograde displacement of fovea.
One of the most frequent complaint is transiently seeing a dark spot in the centre of visual field. The dark spot, which is the subjective representation of a relative scotoma in the centre of the visual ?eld is usually most prominent in the morning immediately after awakening. Patients often report seeing it most clearly when opening their eyes and looking at the ceiling of their bedroom, presumably because the typical ceiling is bright white and unstructured. These characteristics are typical of a relative scotoma, and like the relative scotoma produced by light (i.e. An after-image) it fades within a few seconds, presumably because of theTroxlereffect, a retinal function that subtracts any stationary background stimulus.22
Signs
- Usually a small hyperopic correction can be improved by refraction.
- AC and vitreous are normal.
- Fundus (Slit Lamp Biomicroscopy) shows the following findings:
- Serous retinal detachment:
-
- Round to oval well delineated shallow serous retinal detachment is present in the macula.
- This mildly darkened area is surrounded by a halo of light reflex and has an average size of 2 disc diameters.
- Normal foveal reflex is not apparent.
-
- Serous detachment of RPE:
-
- One or more discrete yellow to yellow grey, round to oval , well demarcated areas of detached RPE maybe observed.
- These areas are often present under the superior half of the macular detachment when gravity forces the SR fluid inferiorly.
- These detachments often less than ¼ of disc diameter in size and have a grayish halo around them.
- Pigment changes maybe present on the detachments surface and occasionally are seen only on an FA.
-
- Sub retinal precipitates:
- Multiple, variably sized yellow dot like precipitates probably caused by SR fluid turbidity maybe noticed at the level of the RPE.
- Diffuse gray, white SR deposits which may represent fibrin are occasionally present.
- There must be a significant hyperpermeability of the choriocapillaris to permit such a large molecule asfibrin(340,000 daltons) to exudate in the extravascular space.
- Eventually the fibrin deposits dissolve in fibrinolysis and disappear; however, in a few cases the deposition of fibrin in the sub retinal space stimulated sub retinal fibrosis and fibrotic scar formation.
- This may cause permanent visual loss and may be complicated by sub retinal neovascularization or vascularization of the fibrous scar and RPE rips.
- In the fovea, a small yellow round spot maybe seen, which maybe caused by increased xanthophyll visibility.
- Extra macular atrophic tracts:
-
- Gass described this as a pseudoretinitis pigmentosa like atypical CSC presentation with prolonged and recurrent serous retinal detachment.
- Frequent in patients of Latin and Asian ancestry.
- Frequent recurrences, permanent visual loss and significant superior visual field loss are common.
- Yannuzzi et al: presumably, a particularly severe or prolonged leakage of fluid or both from RPE defect in SR space at the posterior pole occurs in these patients.
- The SR fluid gravitates inferiorly to form a dependant neurosensory detachment as a flask , tear drop , dumb bell or hour glass pattern.
- Sometimes the tract of SR fluid connecting the macular detachment is so shallow that it is very difficult to appreciate even with the use of fundus contact lenses.
- The RPE under the chronic RD undergoes atrophic changes that appear as atrophic RPE tracts connecting the posterior pole with the dependant RD. Such RPE changes are better noted with FA.
-
- Multiple bullous serous retinal and RPE detachment:
-
- Atypical presentation
- Healthy middle aged men
- Often RD associated with SR fibrinous exudates and multiple serous RPE detachment with areas of shifting SRF
-
- RPE atrophic changes :
-
- Corresponds to previous CSC episodes.
- Visual function and psychophysical tests
-
Functional testing of eyes with serous retinal detachment has demonstrated that: -
- Minimal relative afferent pupillary defect may be present
- Reduced critical ?icker-fusion thresholds
- Prolonged visual evoked potential (VEP) latencies
- Dyschromatopsia
- Depression of central visual ?eld sensitivity.
After resolution,
- The afferent pupillary defect and critical ?icker-fusion thresholds are ?rst to improve.
- Followed by visual acuity, VEP latency and colour discrimination.
- The threshold differential light sensitivity in the central visual ?eld is slowest to improve (Folk et al. 1984).
FFA23
Three types of leakages are seen-
- Smoke stack Pattern.
- Ink Blot Pattern.
- Diffuse Pattern – rare.
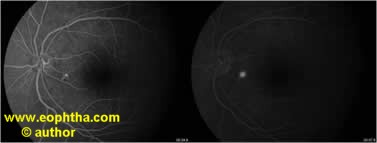
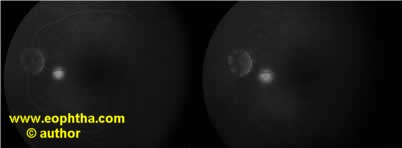
Figure – FFA showing inkblot pattern of leakage
Ink blot pattern
- Seen in 93% cases
- Leakage point/s with uniform dye filling is appreciated.
- Most common location – upper nasal quadrant.
- Least common location – lower temporal quadrant
- Most leakage points are with in 1 mm of fovea but can be till 3 mm of FAZ.
- In recurrent cases, leakage points is with in 1 mm of initial leakage points in 80% cases
- Sometimes the RPED may be present superiorly than the SRD as the fluid collects inferiorly d/t gravity.
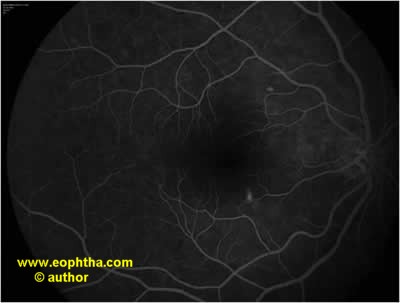
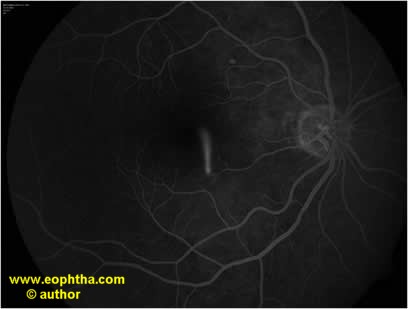
Figure – FFA showing smoke stack pattern of leakage
Smoke stack pattern
- Seen in 7-20%
- Also known asmushroom or umbrella configuration.
- The leakage first ascends superiorly and spreads laterally.
- It is unknown whether the ?ow is caused by a temperature gradient or by a density gradient existing between the newly secreted ?uid and surrounding ?uid that has been in the subretinal cavity long enough to have cooled or to have come hyperdense because of preferential resorption of water and small solutes.
- Detachment associated with a smoke stack type of leakage is larger than that from an active pin point leakage.
Autofluorescence photography24,25
- The auto?uorescence characteristics of the fundus in CSC are clearly differentfrom healthy eyes.
- In acute CSC, hypo?ourescence has been demonstrated at the very point of leakage (Eandi et al 2005). Acute CSC that has persisted for some time often shows granular or semi-con?uent hyper?uorescence throughout the area of detachment.
- In chronic CSC, irregular patterns of mixed hyper- and hypo?uorescence can be seen (Framme et al. 2005; von Ruckmann et al. 2002).
- After reattachment, the auto?uorescent subretinal deposits disappear slowly over a period of several months.
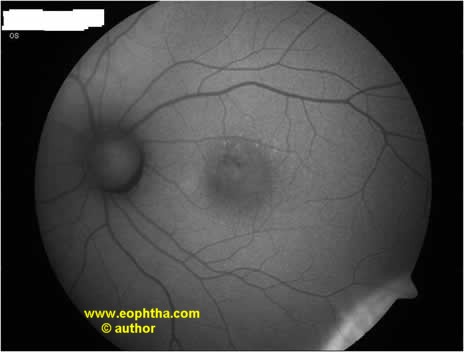
Figure 2 – Fundus Autofluorescence Photograph showing damaged RPE
ICGA26
- The application of ICGA to the study of CSC has expanded the knowledge of the disease.
- Common findings in patients with CSC are multi focal areas of hyperfluorescence in the early and midphases of the study, which then fade in the late phase of the study.
- Typically these areas of hyperfluorescence are found not only in congruence with the leaking point seen with FA, but also in fundus areas that appear clinically and angiographically normal, and in normal fellow eyes of patients with CSC.
- These areas of early hyperfluorescence are believed to represent diffuse choroidal hyperpermeability.
- Another interesting finding with ICGA is the presence of multiple, "occult“, presumed RPE detachments that are imaged with ICGA but are not noted clinically or with FA.
- These areas present the typical ICGA appearance of a serous PED, namely early hyperfluorescence and late hypofluorescence with a rim of hyperfluorescence.
OCT27-31
- It reveals many aspects of pathophysiology of CSC, ranging from subretinal fluid, pigment epithelial detachments, & retinal atrophy following chronic disease.
- It is especially helpful in identifying subtle, even subclinical, neurosensory & macular detachments.
- It is also helpful in identifying the dreaded complication of CNVM in CSC.
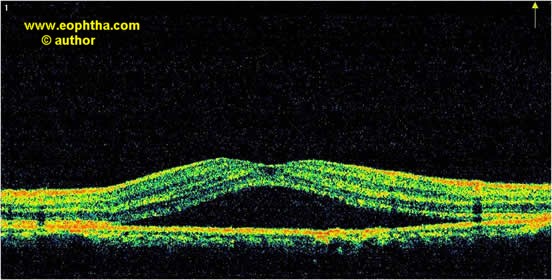
Figure 1 – Spectral Domain OCT showing typical NSR elevation with sub retinal fluid
Multifocal Electroretinogram (mfERG)32,33
mfERG has been used to identify focal regions of decreased retinal function, even in asymptomatic or clinically inactive eyes. Furthermore, investigators, including Lai et al, are using mfERG as a means of assessing the efficacy and safety of new treatment modalities for CSC. During acute CSC, retinal dysfunction is reflected by reduction in mfERG response amplitudes and delay in implicit times. With the use of mfERG, it has also been demonstrated that the felloweye of patients with CSC may have abnormal mfERG responses. It has been shown that mfERG abnormalities may persist even after resolution of the subretinal fluid clinically. Thus, mfERG may therefore have a useful role in providing an objective measure of retinal function in research on the treatment for CSC.
Microperimetry34
Microperimetry-1 (MP1, Nidek technologies) is an instrument for fundus-related perimetry. It captures fundus images of the patient’s retina and at the same time projects light stimuli onto the retina. The light stimuli size have been correlated to Goldmann stimuli sizes (Goldmann I-V) and the pattern are chosen by the operator and can therefore be adapted to different diseases of the macula. The patient’s subjective response to each stimulus (seen/not seen) is recorded (functional information) together with the retinal location of the stimulus (anatomical information). Retinal Microperimetry (MP1) allows an accurate analysis of the central retinal function, combining a digital retinography, a computerized perimetry and a fixation assessment in one exam. In combination with other retinal investigation devices, MP1 has already helped us and will in the future help us to follow and to understand retinal diseases. It has also shown that, despite clinical resolution of CSC, there is lower retinal sensitivity in the macula even once visual acuity returned to 20/20. Fixation studies showed stability of central fixation. Springer et al. investigated patients with Central Serous Choroidopathy (CSC). The MP1 enables quantification of functional defects in patients with CSCR.
Natural History of CSC
- If left untreated - CSC heals spontaneously within 12 weeks with full recovery of VA ~ scar formation.
- Recurrence in 1/2 to 1/3rd Pts is seen
- 3 or more recurrence seen in 10% pts
- Recurrence seen mostly with in 1 yr of disease but may recur up to 10 yrs.
- Even a small single episode of CSC may be followed by chronic slowly progressive disturbances of RPE at post pole.
- Small % age may develop CNV, perifoveal RPE atrophy or cystic macular degeneration with severe and irreversible loss of central vision.
- Larger PEDS may have – multiple recurrence, sub retinal fibrin deposits, multi focal leaks, dependant neurosensory detachment and atrophic RPE tracts.
Treatment
- The high spontaneous remission rate favors conservative management.
- Lifestyle counseling & discontinuation of corticosteroids as first line options.
- If detachment persists for more than 3 months, photocoagulation or PDT should be considered.
- Acetazolamide - promotes the resorption of SRF.
- However, there is no evidence that treatment promotes healing of the RPE lesion, long-term preservation of visual function, or a reduced rate of recurrence.
Laser Photocoagulation
- Has been used for decades.
- Accelerates the resolution of the detachment.
- Also lowers the recurrence rate to about one fifth of what would be expected without active treatment.
- This beneficial effect of photocoagulation treatment can be explained as follows:
-
- The coagulation beam destroys the cluster of diseased pigment epithelium cells, thus stopping the secretion of fluid beneath the neurosensory retina.
- The resulting scar helps to transport fluid back into the choriocapillaris.
Indications
- Persistence of serous detachment for more than 3 months.
- Recurrences in eyes with visual deficit from previous episodes.
- Presence of permanent visual deficit from previous episodes in fellow eye.
- Development of chronic signs i.e, cystic changes in neurosensory retina or widespread RPE abnormalities.
- Occupational or other patient needs that require prompt restoration of vision or stereopsis.
If leakage point is within 500 microns from the center of fovea, wait for 6 months before treating.
Technique
- Strategy is to apply laser energy so as to obtain a confluent coagulation of moderate intensity covering the site of leakage responsible for the foveal detachment.
- Spot size – 200 µ.
- Exposure time – 0.1s.
- End point – bleaching without whitening of outer retina.
- When multiple leaks are present, leakiest one should be treated first.
- Anatomic resolution of the macular detachment generally occurs in about 2 weeks in uncomplicated cases.
- But it may require up to 6 weeks in long-standing detachments with turbid sub retinal fluid.
- Complete visual recovery usually requires twice the amount of time.
Complications
- Worst is, inadvertent photocoagulation of the fovea.
- Persistent scotoma after treatment (should be told to the patient before giving treatment).
- Secondary CNVM.
- Progressive enlargement of the area of RPE atrophy.
Recently, Subthreshold diode laser has been tried in the treatment of ICSC with point source leakage.35
Photodynamic Therpy
Indications: -
- Juxtafoveal lesion.
- Subfoveal lesion.
- Lack of a clearly defined leakage hot spot.
- Concern about the potential induction of CNV.
The use of half-dose verteporfin36 (3 mg/m2) or low fluence PDT (50% reduced light fluence) is done as a precaution against permanent RPE or choriocapillaries damage.
Prognostic Indicator37
Recently Matsumoto H et al has suggested a positive correlation between the ONL thickness with the BCVA in resolved CSC. Discontinuity of the IS/OS line was prevalent in eyes with a thinner ONL and lower BCVA. Despite the good visual outcomes and normal appearance of the IS/OS line, micropsia, and central darkness of the vision often persist in patients with resolved CSC. This phenomenon may be attributed to the decreased cone cells at the fovea.
Table 1. Summary of treatment regimens for CSC
| Duration |
Examination |
Detachment |
Stress, Hypercortisolism |
Treatment |
|
Acute |
Fine granular subretinal deposits if duration more than a few weeks; monofocal leakage |
High bullous OCT > 100 µ subretinal fluid |
Common, recurrent |
Conservative, counseling; if no resolution within 3 months of onset, consider focal photocoagulation if safe, otherwise PDT |
|
Recurrent |
Paucifocal (1-5) |
Moderate 51-100 µ |
Common |
Photocoagulation if safe, otherwise PDT |
|
Chronic |
Multiple semi confluent hypopigmented RPE lesions; confluent subretinal material |
Shallow, often < 50 µ |
Past, current, inconclusive or none |
PDT |
|
Sequelae |
RPE depigmentation without RPE atrophy |
None |
Past, inconclusive or none |
None |
|
Neovascularization |
CNV plus CSC Sequelae; subretinal fibrosis |
Variable mainly around CNV |
History of CSC, associated RPE changes |
PDT &/or intravitreal Anti-VEGF |
References
- Wang M, Munch CI, Hasler PW et al. Central serous chorioretinopathy - Acta Ophthalmol. 2008: 86: 126–145.
- Van Graefe A. Veber Central receidivirende retinitis. Albert Van Graefes Arch Ophthalmol 1866; 12:211-215.
- Klien BA. Macular lesion of vascular orifices: II Functional vascular conditions leading to damage of the macula lutea. Am J Ophthalmol 1953; 36:1-13.
- Maumenee AE. Discussion of Gass FD: Pathogenesis of hemorrhagic disciform lesion of posterior ocular fundus. A histopathologic study. Presented to Wilmer annual meeting, Baltimore, MD, May 26, 1964.
- Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium. II Idiopathic central serous chorioretinopathy. Am J Ophthalmol 1967; 63:587-615.
- Bennet G. Central serous retinopathy. Br J Ophthalmol 1995; 39:605-618.
- Marmor MF. New hypotheses on the pathogenesis & treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol 1988; 226:548-552.
- Spitznas M. Pathogenesis of central serous retinopathy: anew working hypothesis. Graefes Arch Clin Exp Ophthalmol 1986; 224: 321-324.
- Spaide RF, Goldbaum M, Wong DWK et al. Serous detachment of the retina. Retina 2003; 23:820-846.
- Ciardella AP, Borodoker N, Costa DLL et al. The expanding clinical spectrum of central serous chorioretinopathy. Comp Ophthalmol Update 2003; 4:71-84.
- Guyer DR, Yannuzi LA, Slakter JS et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol 1994; 112:1057-1062.
- Ahnoux-Zabsonre A, Quaranta M & Mauget-Faysse M (2004): Prevalence de l’Helicobacter pylori dans la chorioretinopathie sereuse centrale et l’epitheliopathie retinienne diffuse. J Fr ophtalmol 27: 1129–1133.
- Cotticelli L, Borrelli M, D’Alessio AC et al. (2006): Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol 16: 274–278.
- Mauget-Faysse M, Kodjikian L, Quaranta M, Ben Ezra D, Trepsat C, Mion F & Megraud F (2002): Ro ˆle de l’Helicobacter pylori dans la choriore ´tinopathie se ´reuse centrale et l’e ´ pithe ´ liiopathie re ´ tinienne diffuse. J Fr Ophtalmol 25: 1021–1025
- Castro-Correia J, Coutinho MF, Rosas V & Maia J (1992): Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol 81: 379–386.
- Spaide RF, Campeas L, Haas A et al. (1996): Central serous chorioretinopathy in younger and older adults. Ophthalmology 103: 2070–2079.
- Bouzas EA, Scott MH, Mastorakos G, Chrousos GP & Kaiser-Kupfer MI (1993): Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol 111: 1229–1233.
- Carvalho-Recchia CA, Yannuzzi LA, Negrao S, Spaide RF, Freund KB, Rodriguez-Coleman H, Lenharo M & Iida T (2002): Corticosteroids and central serous chorioretinopathy. phthalmology 109: 1834–1837.
- Haimovici R, Gragoudas ES, Duker JS, Sjaarda RN & Eliott D (1997): Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology 104: 1653–1660.
- Haimovici R, Koh S, Gagnon DR, Lehrfeld T & Wellik S (2004): Risk factors for central serous chorioretinopathy: a case–control study. Ophthalmology 111: 244–249. Haimovici R, Rumelt S & Melby J (2003):
- Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology 110: 698–703.
- Iwami S (1995): A new method to elicit pathological entoptic phenomenon from the retina–stenopeic ?icker test. Nippon Ganka Gakkai Zasshi 99: 595–600.
- Gackle HC, Lang GE, Freissler KA & Lang GK (1998): Central serous chorioretinopathy. Clinical, ?uorescein angiography and demographic aspects. Ophthalmologe 95:529–533.
- Eandi CM, Ober M, Iranmanesh R, Peiretti E & Yannuzzi LA (2005): Acute central serous chorioretinopathy and fundus auto?uorescence. Retina 25: 989–993.
- Framme C, Walter A, Gabler B, Roider J, Sachs HG & Gabel VP (2005): Fundus auto?uorescence in acute and chronic recurrent central serous chorioretinopathy. Acta Ophthalmol Scand 83: 161–167.
- Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A & Orlock D (1994): Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol 112: 1057–1062.
- Royce W. S. Chen et al. Speed and Resolution Improve in Newest OCT – Review of Ophthalmology 2007 Pages 84-88.
- Srinivasan V, Wojtkowski M, Witkin A, Duker J, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultra high resolution optical coherence tomography. Ophthalmology 2006;113:2054.
- Wojtkowski M, Srinivasan V, Fujimoto J, Ko T, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2005;112:1734-46.
- Puliafito C, Hee M, Lin C, Reichel E, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995;102:217- 229.
- Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol 2005 89: 562-564.
- Timothy Y.Y. Lai, et al. The Clinical Applications of Multifocal Electroretinography: A Systematic Review - Surv. Ophthalmol 52:61--96, 200
- T. Vajaranant et al. Localized retinal dysfunction in central serous chorioretinopathy as measured using the multifocal Electroretinogram -Ophthalmology, Volume 109, Issue 7, Pages 1243-1250.
- Pascal W. Hasler Microperimetry - a method which combines Perimetry and macular topography - Oftalmolog December 2007.
- Chen SN, Hwang JF, Tseng LF et al. Subthreshold Diode Micropulse Photocoagulation for the Treatment of Chronic Central Serous Chorioretinopathy with Juxtafoveal Leakage Ophthalmology 2008;115:2229–2234.
- Lai TY, Chan WM, Li H, Lai RY, Liu DT & Lam DS (2006): Safety enhanced photodynamic therapy with half dose vertepor?n for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol 90: 869–874.
- Matsumoto H, Taku S, Kishi S. Outer Nuclear Layer Thickness at the Fovea Determines Visual Outcomes in Resolved Central Serous Chorioretinopathy. Am J Ophthalmol 2009;148: 105–110.
