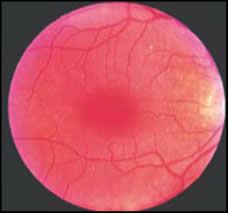
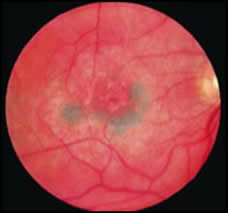
Introduction
Recent advances in the pathogenesis, classification, and surgical intervention of idiopathic macular holes have generated a renewed interest in this entity. Better indicators of the visual outcome, as well as refinements in the surgical technique, have led to improvements in the success of macular hole surgery.
Clinical characterization and theories on the pathogenesis of macular hole have continued to evolve. Although originally thought to be the result of trauma, it is now recognized that most macular holes occur in the absence of antecedent injury and are referred to as idiopathic. Theories for the pathogenesis of idiopathic macular holes have included progressive thinning of the foveal tissue and pre hole cyst formation. The primary pathogenic role of the vitreous was suggested by studies that indicated a low relative risk for macular hole formation in eyes with complete posterior vitreous detachment.
Gass proposed a theory whereby shrinkage of adherent cortical vitreous and subsequent tangential vitreous traction first cause a circumscribed foveolar detachment (stage I) followed by early retinal dehiscence (stage II), then enlargement of the macular hole with vitreofoveal separation (stage III) and finally complete posterior vitreous detachment (stage IV).
Guyer and Green proposed three mechanisms of tangential traction on the macula, including fluid movements and counter-currents, cellular remodeling of cortical vitreous, and contraction of a cellular membrane on the inner surface of the tapered cortical vitreous. Gass emphasized the difficulty in distinguishing posterior vitreous detachment from a zone of posterior vitreous liquefaction and attached posterior cortical vitreous over the macula. He also emphasized that unless the posterior cortical vitreous contains the vitreous condensation ring (Weiss’s ring) over the optic nerve, an operculum, or a pseudo-operculum, the diagnosis of posterior vitreous detachment is uncertain.
Clinical Features
Idiopathic macular holes occur most frequently in the sixth decade of life. According to Gass, stage IA and IB lesions represent focal foveal detachments secondary to vitreous traction. A 100- to 200- um diameter yellow spot is the earliest change observed. With progression, a 200- to 350-um yellowish ring develops. Fine radiating striae are often seen surrounding the yellow ring. The vision is in the range of 20/25 to 20/70. Within several weeks to months, a full-thickness dehiscence develops. This dehiscence often starts eccentrically, and then opens in a “can-opener” fashion to form a crescentic retinal defect, then a horseshoe-shaped hole, and finally a round hole with an operculum. In some cases, the dehiscence starts centrally, with gradual enlargement of the hole, and no operculum develops. A ring of retinal detachment usually surrounds the hole. As the hole enlarges, the vision generally decreases and within several months it progresses to a fully developed hole that measures approximately 500um in diameter. When present, the operculum is suspended over the hole by the detached vitreous cortex. With time, round yellow deposits on the central retinal pigment epithelium, epiretinal membranes that cause contracture of the internal limiting membrane, depigmentation of the pigment epithelium under the cuff of retinal elevation, and a pigmentary demarcation ring defining the outer margin of the retinal detachment may be observed. Vision is usually in the range of 20/70 to 20/400. Posterior vitreous separation from the macula and disk develops in a small percentage of cases.
Eyes with idiopathic macular hole loose vision secondary to tissue dehiscence, cystic changes, and retinal cuff elevation with photoreceptor degeneration. Clinical observations have led to the impression that the macular hole and cuff enlarge secondary to persistent tangential traction from the vitreous, tangential traction from epiretinal membranes and the development of large cystic spaces within the surrounding cuff.
Optical Coherence Tomography
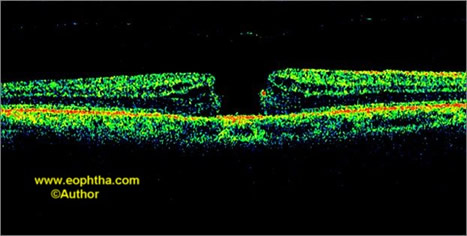
Optical coherence tomography staging is as follows (Figs. 1-4):
Stage 1A: Partial-thickness pseudocyst with perifoveal posterior vitreous detachment
Stage 1B: Full-thickness pseudocyst with roof
Stage 2A: Full-thickness macular hole with the partial opening of the roof, focal vitreous attachment to flap
Stage 2B: Full-thickness operculated macular hole, traction to retina released
Stage 3: Full-thickness operculated macular hole, traction released, ≥400µm diameter
Stage 4: Full-thickness macular hole with complete posterior vitreous detachment, the vitreous face may or may not be evident on optical coherence tomography (OCT).
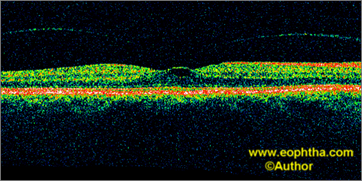
Fig. 1.Stage 1A idiopathic macular hole. OCT highlights perifoveolar posterior vitreous detachment with continued foveolar adherence and obliquely oriented tractional forces. Retinal tissue remains at the base of the pseudocyst.
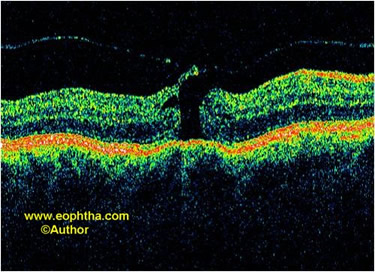
Fig. 2.Stage 2A idiopathic macular hole. The roof of the pseudocyst is torn which continues to have traction exerted by the vitreous attachments.
Fig. 3.Stage 3 idiopathic macular hole. The retinal elements have separated apart and the retina has thickened. An operculum is attached to the visible posterior hyaloid face.
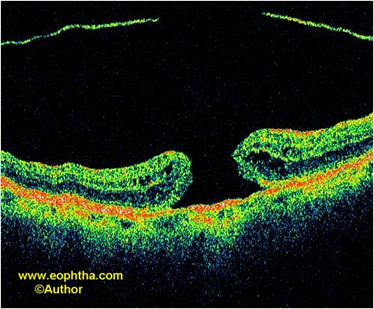
Fig. 4.Stage 4 idiopathic macular hole. The posterior hyaloid face is detached off the surface of the retina.
Spectral-domain three-dimensional imaging of macular holes with high-speed OCT based on SD-OCT technology offers 3-dimensional overviews that facilitate understanding of the abnormalities in the vitreofoveal interface.
It also provides consecutive orthogonal images that allow much more precise and minute observation of 3-dimensionally extending intraretinal structural changes associated with a macular hole than conventional OCT imaging, especially in the photoreceptor inner and outer segments.
Three-dimensional imaging on SD-OCT shows macular hole with altered retinal contour and retinal thickness. Alterations in retinal nerve fiber layer (RNFL) and RNFL-retinal pigment epithelium (RPE) thickness maps without alterations in RPE deformation map are also observed.
Three-dimensional imaging of macular holes with high-speed OCT based on SD-OCT technology offers 3-dimensional overviews that facilitate understanding of the abnormalities in the vitreofoveal interface (Figs. 5 and 6). It also provides consecutive orthogonal images that allow much more precise and minute observation of 3-dimensionally extending intraretinal structural changes associated with a macular hole than conventional OCT imaging, especially in the photoreceptor inner and outer segments.
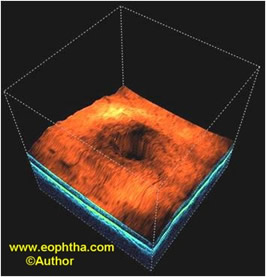
Fig. 5.Idiopathic macular hole. Spectral-domain optical coherence tomography 3D image shows altered retinal contour. Macular hole can be discerned very well.
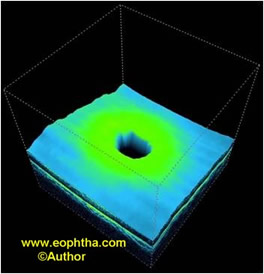
Fig. 6.Idiopathic macular hole. Spectral-domain optical coherence tomography 3D image shows retinal thickness map.
Natural History
The natural history of idiopathic macular hole is well established. Idiopathic macular holes usually lead to a decrease in visual acuity in the range of 20/100 to 20/400.
Histopathologic studies have shown variable photoreceptor degeneration around the hole suggesting the inability to support good vision. However, characterization of visual status using scanning laser ophthalmoscope indicates that good visual function may persist at the edge of the hole.
The Vitrectomy for Macular Hole Study Group reported the baseline characteristics, natural history, and risk factors for progression in eyes with stage II macular holes. Forty-one eyes (37 patients) were analyzed; 19 eyes were randomized to observation (vs. surgery). Mean Snellen visual acuity was 20/66 at baseline. Centric stage II holes usually had a small break (201um average diameter) with a dark yellow ring and without significant retinal elevation. Eccentric stage II holes had a high maximum/minimum diameter ratio and an incomplete cuff of subretinal fluid or yellow ring. Posterior vitreous detachment prevalence was 32% (8/25) and 0% (0/16) in the centric and eccentric hole groups, respectively. The progression rate to stage III or IV was 74%. Progression rate to stage III was 100% in eyes with pericentral hyperfluorescence and 55% in eyes without pericentral hyperfluorescence. Enlargement occurred in 100% of eccentric holes and 60% of centric holes. This group concluded that eccentric and centric holes might have a different pathogenesis. In addition to purely tangential traction, some components of obliquely oriented anteroposterior vitreous traction component may be important for the pathogenesis of senile macular holes, particularly stage II eccentric macular holes. Epiretinal membranes are common in eyes with full-thickness idiopathic macular holes. Although epiretinal membrane prevalence increases with the severity and size of the macular hole, the presence of the epiretinal membrane is not closely correlated with visual acuity. These factors may be important in considering the removal of the epiretinal membrane during vitrectomy for macular hole.
Macular hole reopening is reported to occur in 9.5% of cases. The cause of reopening might have been any anatomic stress such as epiretinal membrane formation or macular edema. However, in most of the reopened cases, no definite cause is evident.
Stage I macular holes can initially be observed. However, excellent visual and surgical results can be obtained in stage I holes with poor vision, or with acute progression to full-thickness holes.
Surgical Case Selection
Many conditions that mimic macular holes have a favorable natural course and require different surgical maneuvers or are not amenable to surgical intervention. Premacular hole lesions are often misdiagnosed and many conditions can masquerade as full-thickness macular holes. An epiretinal membrane with a pseudohole can be confused with a macular hole. The pseudohole usually allows better vision than does the macular hole. In addition, the pseudohole does not have a halo of fluid, an operculum, or yellow deposits at the level of retinal pigment epithelium. A foveal detachment due to central serous retinopathy can be mistaken for a stage Ia macular hole. Both appear as a yellow spot; however, fluorescein angiography can distinguish these two entities. Central serous retinopathy occurs in young to middle-aged men, whereas idiopathic macular holes usually affect elderly women. Cystoid macular edema can also mimic the yellow spot of a stage I lesion. Fluorescein angiography and a history of cataract extraction can be useful in differentiating between these two conditions. The early yellow lesion of solar retinopathy can also appear similar to a stage I lesion. A central drusen or retinal pigment epithelium depigmentation with a small amount of subretinal fluid and a central fibrocellular epiretinal membrane with a macular detachment have been described as mimicking an impending macular hole. The vitreomacular traction syndrome can mimic an impending macular hole. Vitreous traction on the macula due to an incomplete vitreous detachment is responsible for this lesion. These disorders can be distinguished by examination of the vitreous.
A full-thickness macular hole is most accurately diagnosed clinically using a fundus contact lens and slit lamp biomicroscopy. Supplemental tests that can assist in or allow for more accurate diagnosis include Amsler grid testing, testing for a Watzke-Allen sign, and fluorescein angiography. Amsler grid testing is sensitive in detecting any form of macular abnormality, but is not specific enough to be useful in establishing a diagnosis of macular hole, and preoperative testing has not been standardized. The Watzke-Allen test and to a greater degree the laser-aiming beam test further improve the accuracy of diagnosis of full thickness macular holes. The major advantage of these tests is that they are simple to perform, can be done in the office, and are easily accessible. Watzke-Allen sign testing in all patients with clinically defined macular holes shows a break or thinning of the slit beam. Thinning of the beam is seen in both macular hole and pseudomacular hole cases. Therefore, thinning is not as specific as a total break in the slit beam in full-thickness macular hole. The laser-aiming beam test may yield similar diagnostic information, allowing the clinician to test focal areas of the retina for a scotoma. A 50-micron spot laser-aiming beam can be hidden in the macular lesion in all patients with clinically defined full-thickness macular hole. This contrasts with the finding in pseudohole eyes, which could detect the 50 micron spot. In addition, the inability to detect a 200- or 500 micron spot size is noted only by patients with macular holes. Thus, the absolute scotoma detected by the laser beam test is sensitive and specific for full-thickness macular holes. Other ancillary tests, such as focal electroretinography, scanning laser ophthalmoscopy, confocal laser tomographic analysis systems, monochromatic photography, and laser biomicroscopy, have been applied to the study of macular holes with some success. These modalities are not available or feasible for many clinical practices, however. Echographic features of idiopathic macular hole correlate reasonably accurately with clinical features. A pseudo-operculum is a focal condensation of the vitreous cortex suspended on detached invisible posterior hyaloid membrane, in front of either intact foveolar retina or full thickness macular hole. It is demonstrable ultrasonographically. Its presence in front of intact foveolar retina indicates evidence of vitreofoveal separation and low risk of developing a macular hole. Optical coherence tomography has been found effective in distinguishing full-thickness macular holes from partial thickness holes, macular holes, and cysts. It has been successful in staging macular holes and providing a quantitative measure of hole diameter and the amount of surrounding macular edema. It can also detect small separations of the posterior hyaloid from the retina. Careful patient selection is critical to a successful outcome. The ideal candidate would be a patient with bilateral holes of relatively recent onset, with vision in the better eye less than or equal to 20/100. Patients with unilateral symptomatic holes with recently reduced vision to 20/70 or worse are also good candidates. As reported anatomic success rates for macular surgery increase, a method of accurately predicting postoperative visual acuity has increased clinical utility. Both laser interferometer and potential acuity meter have been found to be modestly accurate. Laser interferometer was found to be more accurate in predicting a visual acuity of 20/50 or better.
Long-term follow-up of unoperated macular holes demonstrates a progression in hole size and stage, vision loss which generally stabilizes at the 20/200 to 20/400 level, a redistribution and reduced number of yellow nodular opacities at the level of the retinal pigment epithelium, and the development of retinal pigment epithelial atrophy surrounding the macular hole, resulting in a "bull's-eye" macular appearance.
Management
The new understanding of the pathogenesis of this disorder led to the hypothesis that vision might stabilize or improve if it were possible to relieve the traction, reduce the cystic changes, and reattach the cuff of the detached retina surrounding the hole. The surgical objectives for the repair of macular holes include relief of all tangential traction and retinal tamponade. Identification and removal of the cortical vitreous or posterior hyaloid and removal of fine epiretinal membranes around the hole relieve tangential traction. Tamponade is provided by total gas-fluid exchange with SF6 or C3F8 and strict face-down positioning for at least one week.
Most eyes with macular hole have uniform intraoperative vitreous findings. A zone of collapsed vitreous fibers usually lies anterior to a posteriorly optically clear cavity. In most instances, the vitreous cortex or posterior hyaloid is invisible and remains attached to the underlying internal limiting membrane of the retina. In some cases, the presence of a focally detached vitreous is suggested by an operculum floating above the macular hole.
After surgical removal of the central vitreous, it is necessary to develop and/or complete a posterior vitreous detachment. Using active aspiration (150–250 mm Hg), a silicone-tipped suction cannula is gently swept over the retinal surface near the major arcades or the optic nerve. The area immediately around the hole is avoided. The silicone tip is noted to flex once the cortical vitreous is engaged. This has been termed the “fish-strike sign” or “divining rod sign.” Once engaged, a posterior vitreous detachment can be created by continuous suction with anterior posterior-tangential traction while the tip is moved over the retinal surface. The dissection is carried from the area of initial detachment to adjacent attached areas in an attempt to complete the detachment from the posterior retina to the equatorial zone. Vitrectomy probe with “cutter off” can also be used. The cutter’s large port engages the vitreous more firmly and is more efficient in peeling the cortex from the optic nerve. Once the posterior cortical vitreous is engaged near the optic nerve, it can be peeled from the nerve with continued suction and traction. It is common to create small disk and retinal hemorrhages during this process. Once the vitreous separates from the optic nerve it will usually separate easily to the posterior vitreous base with further gentle traction. Once the posterior hyaloidal dissection has been initiated, the vitreous cortex becomes visible as a thickened translucent sheet, especially the oblique illumination. Occasionally, the disk attachments are so firm that tissue forceps, or pic manipulation is required to complete the posterior vitreous detachment in these areas. A 36-gauge subretinal pic can be useful in engaging the posterior hyaloid near the optic nerve and then pulling off Weiss’s ring. Frequently, an operculum is detected as a glial fragment attached to the vitreous cortex. An operculum or pseudo-operculum can also be detected as a luteal-colored fragment attached to the vitreous cortex. A ring of condensed vitreous (Weiss’ ring) is observed over the disk corresponding to previous vitreopapillary attachment. If the vitreous cortex is not removed it will become apparent during the completion of the air-fluid exchange as a gelatinous substance on the retinal surface.
Fifty percent of operated eyes have some degree of epiretinal membrane proliferation. These epiretinal membranes, unlike typical epiretinal membranes, tend to be finer and more friable, and at times are densely adherent to the retina. The epiretinal membranes may be present surrounding the hole or can involve only a few clock hours. A microbarbed myringotomy blade is used to create an edge in the epiretinal membrane, which is grasped with tissue forceps and stripped. One disk area around the macular hole is checked and liberated from epiretinal membranes to ensure the relief of traction. During this maneuver, it is common to create small hemorrhages around the hole. Damage to the inner retina is avoided, an early sign of which may be the development of fluffy whitish areas. Prolonged intense illumination from the light pipe near the macula is avoided to prevent phototoxicity. A total air-fluid exchange is performed and effort is made to dehydrate the vitreous cavity. The shallow fluid in the base of the optic disk cup is aspirated repeatedly, with a soft-tipped cannula, until fluid no longer collects. A non-expansive concentration of long-acting gas is exchanged for air. Postoperatively, strict prone positioning is prescribed. At the 1-week visit if the edges of the macular hole are flattened and imperceptible with flattening of the cuff of retinal detachment, anatomic success is assured (Figs. 7-10). However, if the edges are still visible and the cuff elevated, anatomic failure is probable.
Internal Limiting Membrane Peeling
The rationale for internal limiting membrane peeling is as follows:
-Relaxation of tangential traction of internal limiting membrane itself
-Removal of possible cause of persistent fine retinal fold
-Removal of epiretinal proliferation
-Removal of diffusion barrier
-Differences between internal limiting membrane and retina
Surgical Technique
Internal Limiting Membrane Peeling
The first step is puncturing the internal limiting membrane with a sharp-tipped and barbed microvitreoretinal blade in the superior macula about half-disk diameter away from the macular hole. Once the internal limiting membrane is punctured, the microvitreoretinal blade is drawn gently across the surface of the retina for a short distance until a small opening is created. There is typically a white “fluffy” appearance to this opening and occasionally a fleck of hemorrhage. A pic is advanced and wiggled slightly from side to side in an effort to engage only the internal limiting membrane and not the nerve fiber layer. Once a proper dissection plane is established, the effort is made to tunnel beneath the internal limiting membrane in a counter-clockwise direction, creating a pocket to work in. Intermittent slight lifting motion is used to peel the internal limiting membrane off the nerve fiber layer ahead of the instrument tip. The internal limiting membrane is often not visible over the instrument tip, but a slight movement of the retina in the direction of the pic indicates that it has been engaged. Once the internal limiting membrane is elevated this extent, it can be grasped with an internal limiting membrane or end-gripping forceps and peeled around the macular hole.
Indocyanine Green-assisted Internal Limiting Membrane Peeling
Indocyanine green dye is prepared as follows:
(a) 25 mg of indocyanine green dye is diluted with 0.5 ml of distilled water. Then 4.5 ml of balanced salt solution (BSS) is added to make 0.5% solution.
(b) 25 mg of dry indocyanine green substance is first dissolved with 5 ml sterile distilled water. One milliliter of this solution is then diluted with 9 ml of BSS Plus. Indocyanine green with a concentration of 0.05% is then applied to stain the internal limiting membrane. 0.2 to 0.5 ml of diluted indocyanine green is placed/sprayed over the retina under normal infusion. The dye is washed out immediately. After removal of the indocyanine green, internal limiting membrane peeling is performed.
internal limiting membrane peeling can be done as follows:
(1) Directly grasping the internal limiting membrane by forceps.
(2) Creating a tear/rent in the internal limiting membrane by:
A. Barbed microvitreoretinal blade or bent 28-gauge needle
B. Tano’s diamond-dusted membrane scraper: It is a useful tool for membrane separation during vitreous surgery. A diamond-dusted silicone cannula is fashioned from flexible silicone tubing with a beveled tip and coated with diamond fragments. It has been found to be particularly useful for removing residual vitreous cortex and epiretinal membranes from around the hole. It is also effective in removing "immature membranes" in proliferative vitreoretinopathy. A diamond-dusted silicone cannula is a useful tool for removing thin epiretinal membranes and vitreous cortex that may be difficult or nearly impossible to remove safely using other techniques.
C. Silicone brush
D. Suction with a back-flush needle
(3) Completion of internal limiting membrane peeling:The unstained, underlying retina is readily apparent in contrast to the green-stained internal limiting membrane. Subsequently, the flap of the internal limiting membrane is easily grasped and peeled with a diamond-dusted intraocular forceps. Continuous curvilinear maculorhexis is performed. The contrast enables the surgeon to monitor precisely the extent and the completeness of the internal limiting membrane peeling. Subtle frills of the internal limiting membrane are not left at the edge of macular hole. The bare area free of the internal limiting membrane is intended to be one disk diameter surrounding the macular hole. Persisting autofluorescence of the macular area or the optic nerve is observed up to 3 months after surgery.
Infracyanine Green-assisted Internal Limiting Membrane Peeling
To prepare the infracyanine green solution (S.E.R.B., Paris, France), a 25- mg vial of infracyanine green is dissolved in 5 ml of glucose 5% solution to obtain final infracyanine green concentration of 0.5% with an osmolarity of 309 mOsm/kg. After closure of the infusion line, 0.2 ml of this solution is instilled directly into the posterior vitreous cavity over the macula using a blunt cannula. After 2 minutes, the infusion line is reopened and the excess of dye was removed from the vitreous cavity using a backflush cannula. The internal limiting membrane that had been in contact with the dye is stained green diffusely.
Trypan-blue assisted Internal Limiting Membrane Peeling
0.5 to 1 ml of trypan blue 0.06% (Visionblue, DORC International, The Netherlands) is injected under continuous infusion over the posterior pole under direct visualization, staining the internal limiting membrane. Alternatively, after the fluid-air exchange, the dye is placed over the macula under air sufflation. Air fluid-exchange is done to aspirate the dye. A 20-gauge bent microvitreoretinal blade is then used to incise the membrane, and diamond-dusted intraocular forceps are used to remove it in a circumferential manner 360 degrees around the macular hole
Triamcinolone-assisted Vitrectomy
The preservative of triamcinolone acetonide is removed with a filter and rinsed with the balanced salt solution. The triamcinolone acetonide particles (40 mg) are then resuspended in 2 ml of balanced salt solution. Following core vitrectomy, triamcinolone acetonide suspension is injected over the posterior pole. The posterior hyaloid is clearly observed. Separation of the hyaloid from the optic nerve head and posterior retina is then performed. Next, a subtotal vitrectomy is performed. The triamcinolone acetonide suspension is injected again over the posterior pole, and excess triamcinolone acetonide is aspirated with a backflush needle. Numerous particles of triamcinolone acetonide are observed as white specks on the posterior retina. The internal limiting membrane is then grasped with an intraocular forceps and peeled in a circumferential manner around the macular hole. The peeled area is clearly observed as an area lacking the white specks left by the triamcinolone acetonide. Several particles of triamcinolone acetonide are observed on the peeled internal limiting membrane. Triamcinolone acetonide tends to stick to the residual vitreous cortex on the internal limiting membrane or to the internal limiting membrane itself. The peeled area of the membrane is clearly confirmed by the lack of white specks left elsewhere by the triamcinolone acetonide. The internal limiting membrane can be easily peeled in a circumferential manner as using indocyanine green. A small amount of triamcinolone acetonide often deposits in the macular hole, however. Local toxicity of triamcinolone acetonide to the neural retina and retinal pigment epithelium is unknown.
Perfluorocarbon liquid-assisted Internal Limiting Membrane Peeling in Macular Hole associated with Detachment
Stripping the internal limiting membrane is supposed to be beneficial in attaching the retina in retinal detachment because of the macular hole. However, it is difficult to lift up and strip the internal limiting membrane over extensively detached retinas because such retinas are very mobile. To evade this difficulty, flattened the detached retina can be flattened with perfluorocarbon liquid and peeling of the internal limiting membrane is done in the presence of perfluorocarbon liquid. The internal limiting membrane flap is easily turned up and enlarged with the posterior counter traction by perfluorocarbon liquid.
In another technique, the subretinal fluid is drained with the vitreous probe held over the macular hole and operating in the aspirating mode (200 mm Hg). Once the macular hole has flattened, the infusion is stopped, and perfluoro-n-octane is injected slowly as a single large bubble over the posterior pole. Following the injection and gradual retinal attachment, indocyanine green (0.5%, 270 mOsm) is instilled (approximately 2 ml of the solution) over the macula at the retina–perfluoro-n-octane interface. The infusion is reopened following indocyanine green injection, and the intravitreal dye is washed out. The dye between the perfluoro-n-octane bubble and the retina is then flushed with an injection of balanced salt solution at the perfluoro-n-octane–retina interface using a soft-tip needle attached to a syringe. The internal limiting membrane is stained green and became clearly visible under the perfluoro-n-octane. The dye also stained the retinal pigment epithelium within the hole. Passage of the dye into the subretinal space does not occur. The internal limiting membrane is removed using standard micro end-gripping forceps.
Tamponade
Gas:A non-expansive concentration of long-acting gas (C3F8) is exchanged for air. Postoperatively, 24 hour-a-day strict prone positioning for 5-7 days is prescribed. Face down positioning increases the buoyancy effect of gas bubble, which may augment the surface tension effects of intraocular gas tamponade, which in turn may enhance the anatomic closure of macular holes.
Silicone Oil:Silicone oil eliminates the need for face-down positioning allows immediate return of normal functioning and unrestricted air travel and possibly eliminates visual field defects. It requires a second procedure to remove it after 2-3 months, and is possibly not as effective as gas tamponade. The rate of hole closure is significantly lower than for C3F8 gas. Silicone oil may be considered if: the patient is either unable or unwilling to maintain face-down position postoperatively, early return to normal activity is necessary, patient is monocular and postoperative air travel is required.
F I G U R E 7. Preoperative stage II macular hole. Watzke-Allen sign is present.
F I G U R E 8. The postoperative fundus photograph shows a closed macular hole.Visual acuity is 20/25-1.Watzke-Allen sign is absent.
The etiology of anatomic failure is uncertain. Patient noncompliance in postoperative prone positioning and subsequent inadequate tamponade, production of traction by residual epiretinal membranes, intrinsic retinal changes that cause stiffness, and prevention of retinal reattachment possibly plays a role.Experience with reoperation has been limited. If failure is believed to be secondary to residual epiretinal membranes, reoperation has been successful. If noncompliance with postoperative prone positioning is thought to be the cause of failure, newly motivated patients can be given a second chance. Macular holes can reopen after initial surgical repair. Those cells that can lead to the closure of an idiopathic macular hole can also contribute to its recurrence if the reparative process goes awry.
Macular holes of > or = 2 years' duration may be more difficult to close successfully than are more recent macular holes, and the visual improvement appears to be less favorable. Many eyes with chronic macular holes gain substantial visual acuity, so vitreous surgery can be considered in selected eyes with chronic macular holes based on visual needs.
Although vitrectomy is a risk factor for nuclear sclerosis progression, the duration of vitrectomy does not increase the risk.
Patients with chronic systemic illness such as arthritis may be unable to carry out this postoperative regime. Thus there has been a need for alternative techniques that would eliminate such a regime. In patients who underwent macular hole surgery using silicone oil without any postoperative posturing, the anatomical results have been reported to be similar in 80% cases in keeping with those reported in other studies using gas tamponade. However, the visual results are disappointing and less rewarding than those obtained after successful surgery using gas tamponade.
Visual acuity in patients after anatomically successful macular hole surgery continues to improve even beyond 1 year after surgery. Although substantial improvement occurs soon after cataract extraction, further improvement in visual acuity continues for 2 years thereafter. The retinal pigment epithelium alterations and retinal detachments are common after macular hole surgery and result in significantly reduced postoperative visual acuity.
According to Tornambe and associates successful macular hole closure is possible without face-down positioning. This technique may be an alternative for patients with macular holes in pseudophakic eyes that are unable to assume face-down posturing. Combining cataract surgery with this technique for macular hole repair is reasonable for phakic patients who cannot maintain prone positioning. Major disadvantages of combined surgery include the morbidity of the second procedure and removal of a visually insignificant cataract. This approach should be considered for those patients unable to tolerate face-down positioning.
After macular hole surgery, anatomically unsuccessful closure of the hole correlates with small enlargements in the diameter of the macular hole and its surrounding subretinal fluid cuff, and with a slight decrease in visual acuity. Macular hole closure after repeat surgery improves visual acuity outcome in the majority of retreated eyes.
F I G U R E 9.Preoperative stage III macular hole. Visual acuity is 20/200.
F I G U R E 10. Post-operative stage III macular hole. Visual acuity is 20/40.
Complications
The most common complication of a vitrectomy for impending macular hole and a full-thickness macular hole is the occurrence or progression of nuclear sclerotic cataracts. Of the patients treated for macular holes, 20% to 33% require cataract extraction postoperatively Nuclear sclerotic cataracts progress substantially after macular hole surgery with long-acting intraocular gas tamponade.
Park and associates noted posterior segment complications in 23% of their cases. These included peripheral retinal breaks (3%), rhegmatogenous retinal detachment from a peripheral retinal break (14%), enlargement of the hole (2%), and late reopening of the hole (2%), retinal pigment epithelium loss under the hole (1%), photic toxicity (1%), and endophthalmitis (1%). Iatrogenic retinal breaks tend to be in the inferior and temporal retina, which establishes the need for greater intraoperative surveillance in these areas. Peripheral retinal tears may develop during stripping of cortical vitreous. The free edge of the macular hole is mobile and susceptible to incarceration during aspiration maneuvers, which can lead to tearing and enlargement of the hole. During membrane stripping or fluid aspiration care must be taken to avoid excessive traction and inadvertent damage to the edge of the macular hole and inner retina. Retinal pigment epitheliopathy after macular hole surgery may portend a guarded visual prognosis in affected patients undergoing successful macular hole repair (Figure 11). This may be the result of individual patient sensitivities to manipulation, direct trauma, or prolonged exposure to the endoilluminator. Poliner and Tornambe hypothesized that combination of prolonged intraocular gas contact and light exposure exceeding threshold for an already compromised macula appear to be responsible for this pigmentary pattern. According to Charles, retinal pigment epithelium changes seem more likely to be secondary to trauma to the retinal pigment epithelium and photoreceptors and occur secondary to precipitous suction removal of thick subretinal fluid through macular holes. Duker hypothesized that the persistence of subretinal fluid may be as important as individual susceptibility or overall light exposure for the development of this epitheliopathy.
Late reopening can complicate initially successful macular hole surgery and may occur in at least 4.8% of initially successful operations. The reopening has been documented to occur at between 2 and 22 months, and it has been hypothesized that the growth of an epiretinal membrane plays a part in at least some of the eyes. Repeat vitrectomy with gas injection can result in re-closure of the hole and improvement in vision. Kokame reported late recurrence of macular hole in an eye with impending macular hole, which initially resolved after surgical intervention. The mechanism of late recurrence may be similar in impending and full-thickness macular holes. Smiddy also noted macular hole development after surgical peeling of epiretinal membrane over the macula. Thus macular holes can develop in certain situations in which the posterior cortical vitreous has been removed from the macula, either surgically or by natural posterior vitreous detachment.
F I G U R E 11.Retinal pigment epitheliopathy following vitrectomy and gas-fluid exchange for stage IV macular hole.Although the hole is closed, the patient sees a ring scotoma, and visual acuity is 20/150.
Holekamp and associates reported ulnar neuropathy as a complication of macular hole surgery.
A significant temporal field defect may occur in patients after otherwise uncomplicated surgery for macular holes. The cause is unclear; however, reduction in nerve fiber layer thickness from the superior and nasal peripapillary area suggests that acute surgical release of the posterior hyaloid and the use of long-acting intraocular gas may in certain patients result in visual field defects. The most common visual field defect is dense and wedge-shaped and involves the temporal visual field. Although unclear, the etiology may involve trauma to the peripapillary retinal vasculature or nerve fiber layer during elevation of the posterior hyaloid or during aspiration at the time of air-fluid exchange, followed by compression and occlusion of the retinal peripapillary vessels during gas tamponade. Visual field defects can occur following vitrectomy and gas-fluid exchange for macular hole. Two categories of scotomas have been observed: peripheral and relative arcuate. The cause of peripheral visual field loss is unclear. Increased intraocular pressure may be the cause of relative arcuate scotomas. Peripheral visual field defects after macular hole surgery can be a complication of very low incidence. A rather low-pressure set during air-fluid exchange as well as special aspects of the surgical technique may be responsible for this low incidence of peripheral visual field defects. Small, mostly asymptomatic, paracentral scotomata as a complication after vitrectomy for idiopathic macular hole have not been reported in the literature so far. Whether they are caused by trauma to the nerve fibers during surgery or other factors remain unknown. Fundus changes become apparent after surgery, and they are progressive. Therefore, it is important to examine eyes with visual field defects for a follow-up period of several years. The location of the visual field defect correlated with the location of the infusion cannula. The incidence of this visual field defect was influenced strongly by the infusion air pressure. The visual field defect may be caused by the mechanical damage of air infusion. Dehydration injury of the nerve fiber layer during the fluid-air exchange should be considered as a possible cause of visual field defect after pars plana vitrectomy for macular hole. Passing air used for fluid-air exchange through water seems to prevent visual field defects after vitrectomy for macular hole surgery. Visual field defects that occur after room air is used may result from desiccation of the retina by room air.
Some eyes develop increased intraocular pressure after vitreous surgery for macular hole, and the increase occurs most frequently between two days and two weeks postoperatively.
Internal Limiting Membrane Peeling Complications
Intravitreal indocyanine green-assisted internal limiting membrane peeling improves anatomic success in macular hole surgery, but it may potentially lead to unfavorable visual acuity outcome and peripheral visual field loss. Fundus fluorescence is observed after indocyanine green. Smiddy and associates did not find internal limiting membrane peeling essential in macular hole surgery.
Retina exposed to indocyanine green concentrations used in human vitreoretinal surgery had greater retinal pigment epithelial atrophy and outer retinal degeneration than control eyes undergoing the same surgery without indocyanine green. Eyes filled with infusion fluid during indocyanine green injection had less damage to the retinal pigment epithelium and outer retina than did air-filled eyes receiving indocyanine green. Potential damage to the neurosensory retina is associated with the intraoperative administration of the indocyanine green solution. Whether toxic effects of the dye itself cause this, mechanical trauma to the retina or other mechanisms remains unknown. It has also been suggested that intravitreal application of indocyanine green may cause retinal damage by altering the cleavage plane to the innermost retinal layers. That may result in less improvement of visual acuity and unexpected visual field defects. The underlying mechanisms of action remain unclear.
Macular hole surgery with peeling of the internal limiting membrane without the use of adjuvants or internal limiting membrane staining leads to good functional long-term results. Paracentral scotomata remain subclinical in most cases and may be due to a mechanical trauma of the nerve fiber layer. Anatomical changes of the macula following vitrectomy with removal of the internal limiting membrane are infrequent. However, paracentral scotomata observed in our series might be caused by a trauma to the nerve fibers during internal limiting membrane peeling. Altered uptake of infrared diode laser by retina after intravitreal indocyanine green dye and internal limiting membrane peeling has been reported. Indocyanine green and intense light exposure in retinal pigment epithelium cells causes photosensitizing toxicity that is reduced when sodium in the solvent is eliminated and replaced with other cations. Eliminating sodium from the solvent reduces indocyanine green uptake into retinal pigment epithelium and its associated photosensitizing toxicity. This reconstitution method of indocyanine green may be helpful for safer intravitreal indocyanine green use in macular hole surgery.
Dilutions of indocyanine green as recommended in the literature may alter the structure of the retina to some degree. Possible factors responsible for this inadvertent action may include:
(1) concentration,
(2) osmolarity pH,
(3) time of tissue contact, and
(4) mechanical factors from more forceful traction during peeling.
The use of silicone oil in macular hole surgery with internal limiting membrane peeling may complicate the postoperative outcome. Internal limiting membrane defects may facilitate the entry of silicone oil into the retina, leading to accumulation of oil vacuoles. Infracyanine green-assisted removal of the retinal internal limiting membrane appears to induce a high incidence of anatomical closure, with good visual outcome.
Miscellaneous
Combining cataract surgery with vitrectomy surgery may prevent a later second operation for post-vitrectomy cataract formation. Biometry after macular hole surgery should be corrected by subtracting the depth of the foveolar crater (0.5 mm, the estimated depth of the foveolar crater) from the measured axial length. C3F8 gas has proved to be a more effective tamponade than silicone oil with respect to achieving initial closure of macular holes. Eyes receiving an oil tamponade required significantly more reoperations to achieve a similar rate of hole closure compared with eyes undergoing a gas tamponade. Final visual acuity was better for gas-operated eyes than for silicone-operated eyes.
Perfluorohexyloctane merits further evaluation for ocular endotamponade in patients with persisting macular holes.
Role of Optical Coherence Tomography
Optical coherence tomography plays an important role in the diagnosis and management of macular hole. According to Tornambe optical coherence tomography images and a simple model suggest macular hole formation may be due to a defect in the inner retina with secondary vitreous fluid accumulation into the middle and outer retinal tissue. Idiopathic macular holes have one of two patterns early after surgical closure, simple closure or a bridge formation. Visual improvement starts after the fovea assumes a normal configuration. The bridge formation appears to reflect an early phase and fragile condition in the anatomic closure of macular holes. A small macular hole appears to be closed by 3 days after vitrectomy with gas tamponade. Although we cannot generalize to all sizes of macular hole, our findings suggest that small macular holes are closed much earlier than reported and that the duration of maintaining a prone position can be shortened. There is a close correlation between the stage of the macular hole and the degree of posterior vitreous detachment.
This close correlation suggests that progression of idiopathic macular hole is related to enlargement of the posterior vitreous detachment. Ullrich and associates assessed macular holes according to the classification by Gass with optical coherence tomography before pars plana vitrectomy. Macular hole diameters were determined at the level of the retinal pigment epithelium (base diameter) and at the minimal extent of the hole (minimum diameter). Calculated hole form factor (HFF) was correlated with the postoperative anatomical success rate and best corrected visual acuity. The duration of symptoms was correlated with base and minimum diameter of the macular hole. In eyes without anatomical closure of the macular hole after one surgical approach the base diameter and the minimum diameter were significantly larger than in cases with immediate post surgical closure. There was a significant negative correlation between both the base and the minimum diameter of the hole and the postoperative visual function. In all patients with HFF >0.9 the macular hole was closed following one surgical procedure, whereas in eyes with HFF <0.5 anatomical success rate was 67%. Better postoperative visual outcome correlated with higher HFF. Preoperative measurement of macular hole size with optical coherence tomography can provide a prognostic factor for postoperative visual outcome and anatomical success rate of macular hole surgery. The duration of symptoms did not correlate with the diameters measured. Base and minimum diameters especially seem to be of predictive value in macular hole surgery. Visual outcome after anatomic closure of macular holes by vitrectomy is closely related to the structure of the center of the fovea postoperatively.
The repaired macular holes were classified by the optical coherence tomography images by Uemoto and associates as being of "good shape" (nearly normal foveal contour) or "poor shape" (abnormal foveal contour with flat fovea and steep edge or with a thick retina without a foveal pit). Internal limiting membrane peeling may provide better anatomical success and recovery of the macular shape, but the postoperative visual acuity and improvement of visual acuity were not related to the morphological results.
Optical coherence tomography findings of postoperative macular hole closure status correlate well with the clinical findings. Careful clinical examination alone may be adequate in determining the surgical anatomical end points in the majority of patients after macular hole surgery (Fig 12).
The postoperative closure of idiopathic macular holes following vitreous surgery was related to the preoperative macular hole diameter determined by optical coherence tomography, with lesions smaller than 400 micron demonstrating higher success rates. A trend toward greater visual acuity improvement was demonstrated for idiopathic macular holes smaller than 400 micron. Late reopening was only seen in macular holes that were 400 micron or larger measured by optical coherence tomography. Preoperative analysis and measurement of idiopathic macular holes with optical coherence tomography may help delineate postoperative expectations for successful anatomical closure of the macular hole, visual acuity, and long-term closure.
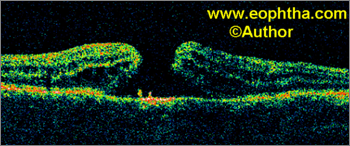
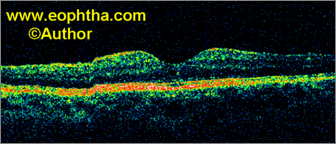
Fig. 12. Macular Hole Surgery: Optical coherence tomography. Optical coherence tomography shows a full thickness macular hole. Intraretinal changes of neurosensory retina at the hole edge are observed. Postoperative optical coherence tomography after successful macular hole surgery shows well-maintained foveal contour.
Vitrectomy surgery for impending macular hole based on optical coherence tomography has also been suggested. It is possible to repair macular holes by using optical coherence tomography to guide the dissection of the vitreous from the macular hole followed by limited vitrectomy. By using a less invasive approach, it may be possible to repair macular holes in less operative time and with fewer complications. A microspatula knife is used to dissect the connection between the vitreous and the retina previously delineated by optical coherence tomography. The posterior vitreous was not stripped from the retinal surface. Limited vitrectomy over the hole was performed to create a space for a gas bubble.
References:
1. Gass JDM, Johnson RN. Idiopathic macular holes. Observations, stages of formation, and implication for surgical intervention. Ophthalmology.1988;95:912–924.2. Gass JDM. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752–759.
3. Kim JW, Freeman WR, El-Haig W, et al, the Vitrectomy for Macular Hole Study Group. Baseline characteristics, natural history, and risk factors to progression in eyes with stage II macular holes. Results from a prospective randomized clinical trial. Ophthalmology. 1995;102:1818–1829.
4. Watzke RC, Allen L. Subjective slit lamp beam sign for macular disease. Am J Ophthalmol. 1969;68:449–453.
5. Martinez J, Smiddy WE, Kim J, Gass JDM. Differentiating macular holes from macular pseudoholes. Am J Ophthalmol. 1994;117:762–767.
6. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748–756.
7. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–659.
8. Wendel RT, Patel AC. Full-thickness macular hole. In: Bovino JA, ed. Macular surgery. Norwalk, CT: Appleton & Lange, 1994:49–60.
9. Kim JW, Freeman WR, Azen SP, et al. Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Vitrectomy for Macular Hole Study Group. Am J Ophthalmol. 1996;121:605-614.
10. Wendel RT, Patel AC, Kelly NE, et al. Vitreous surgery for macular holes. Ophthalmology. 1993;100:1671–1676.
11. Banker AS, Freeman WR, Kim JW, et al. Vision-threatening complications of surgery for full-thickness macular holes. Vitrectomy for Macular Hole Study Group. Ophthalmology. 1997;104:1442-1452.
12. Park SS, Marcus D, Duker JS, et al. Posterior segment complications after vitrectomy for macular hole. Ophthalmology. 1995;102:775–781.
13. Sjaarda RN, Glaser BM, Thompson JT, et al. Distribution of iatrogenic retinal breaks in macular hole surgery. Ophthalmology. 1995;102:1387–1392.
14. Poliner LS, Tornambe PE. Retinal pigment epitheliopathy after macular hole surgery. Ophthalmology. 1992;99:1671–1677.
15. Eckardt C, Eckardt U, Groos S, et al. Removal of the internal limiting membrane in macular holes. Clinical and morphological findings. Ophthalmologe. 1997;94:545-551.
16. Gass CA, Haritoglou C, Schaumberger M, Kampik A. Functional outcome of macular hole surgery with and without indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2003;241:716-720.
17. Haritoglou C, Gass CA, Schaumberger M, et al. Long-term follow-up after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol. 2002;134:661-666.
18. Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120:29-35.
19. Saxena S. Focus on macular diseases. Jaypee medical publishers, New Delhi. 2006.
20. Saxena S, Meredith TA. Optical coherence tomography in retinal diseases. Jaypee medical publishers, New Delhi. 2005.