Limbal Epithelial Stem Cells
Anatomy:
The healthy corneo-scleral limbus (Figure 1) is a gradual transition zone from the stratified, non- keratinised squamous epithelium of the cornea to the stratified, non- keratinised columnar epithelium with mucin-secreting goblet cells of the conjunctiva. It has 7-10 layers of cells, which have attachments similar to the corneal cells.
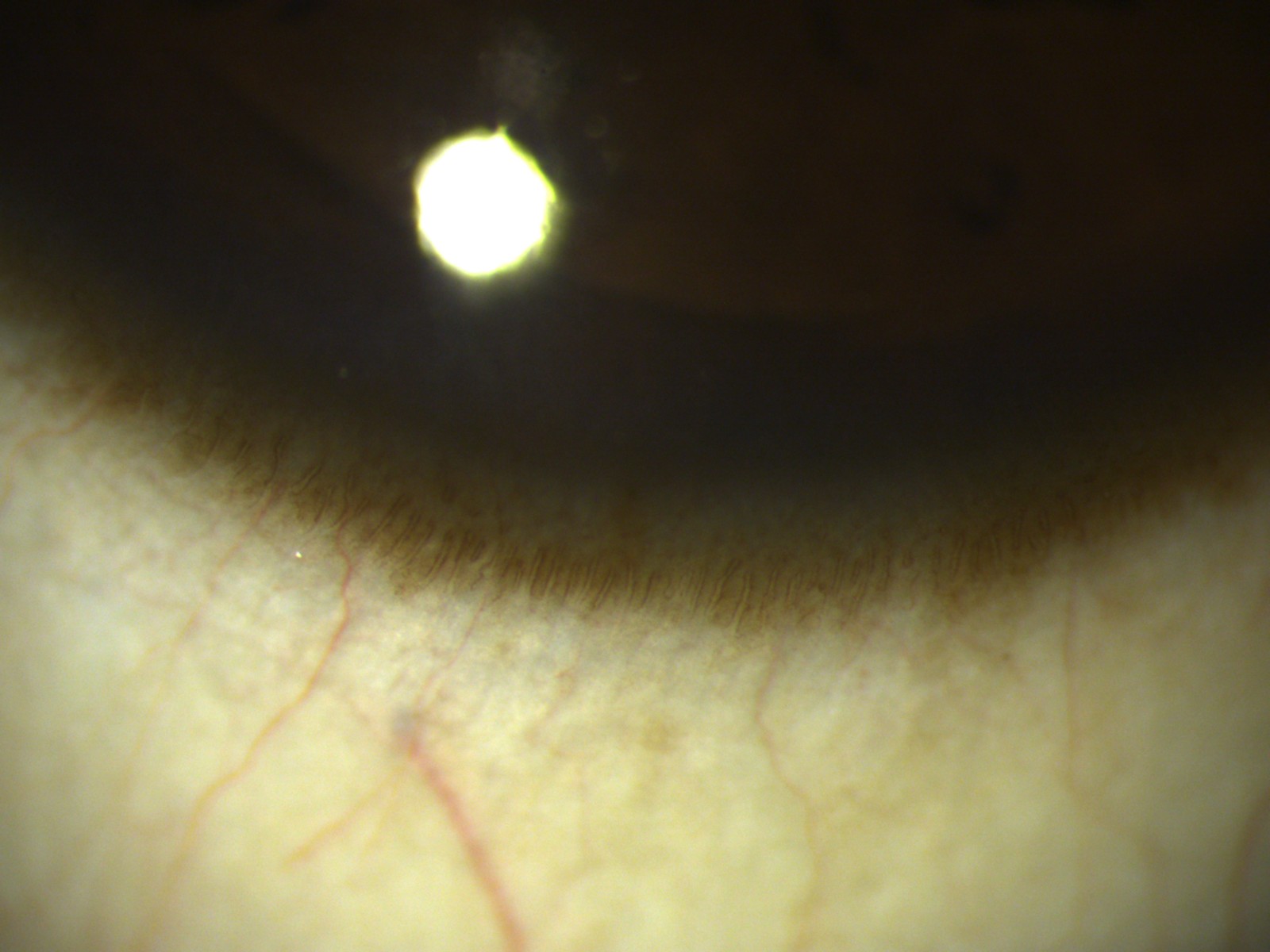
This zone contains limbal epithelial stem cells (LSCs) that generate new epithelial cells during homeostasis and after injury or insult, to maintain corneal transparency and vision.
LSCs are believed to be located within the basal layer of limbal crypts (in the palisades of Vogt) [1] and focal stromal projections (finger-like projections of stroma containing a central blood vessel, and extend upward into the corneal limbal epithelium and are surrounded by small, tightly packed basal cells). The presence of a dedicated vasculature network and dense innervation suggests that this is an essential component of the niche environment, supplying nutrients and possibly survival factors to LSCs [2]. The limbal crypts predominantly occur on the superior and inferior cornea where they are normally covered by the eyelids, thus providing a protective environment for LSCs. The deep undulations of the Palisades of Vogt at the limbus provide LSC with an environment that protects them from shearing forces. The palisades are mostly highly pigmented with melanocytes, to shield LSCs from damaging ultraviolet light and the resultant generation of reactive oxygen species.
Function:
A fine balance between cell proliferation, differentiation, migration, and apoptosis is necessary.Thoft and Friend [3] proposed this "X, Y, Z hypothesis of corneal epithelial maintenance": As the LSCs divide, one of the daughter cells retains the primitive nature of the parent cell and functions to maintain the stem cell pool. The other daughter stem cell differentiates to acquire the physiological nature of the corneal epithelium. These cells are known as ‘transient amplifying cells, which then divideand migrate vertically, circumferentially, and centripetally from the basal layer to replace the surface epithelial cells that are desquamated. Once fully differentiated, the squamous cells are shed from the ocular surface during normal wear and tear and this, in turn, stimulates the cycle of cell division, migration, and differentiation. The tear film, aqueous humour, keratocytes, and epithelial cells themselves provide a variety of cytokines and growth factors that play important roles in the maintenance and wound healing of the cornea.
Detection Techniques:
Characteristics of limbal stem cells include a slow turnover rate (therefore retain DNA labels for long time periods), high proliferative potential (in the event of injury), clonogenicity, expression of stem cell markers, as well as the ability to regenerate the entire corneal epithelium.LSCs share common features with other adult somatic stem cells including small size and high nuclear to cytoplasmic ratio. The putative LSC markers ABCG2 and p63α are expressed only by limbal basal cells and not by the corneal epithelium. They also lack expression of differentiation markers such as cytokeratins 3 and 12.
DEFINITION OF LSCD:
LSCD is an ocular surface disease caused by a decrease in the population and/or function of corneal epithelial stem/ progenitor cells; this decrease leads to the inability to sustain the normal homeostasis of the corneal epithelium [4].
The disease is characterized by conjunctivalization (i.e., replacement of the normal corneal epithelium by conjunctival epithelium) and/or other signs of epithelial dysfunction such as persistent or recurrent epithelial defects with or without neovascularization, ocular surface inflammation, and scarring. Frequent consequences are decreased vision and discomfort, leading to reduced health-related quality of life. LSCD may present alone as a single entity or associated with abnormalities of other components of the ocular surface such as the conjunctiva, meibomian glands, lacrimal glands, tears, corneal nerves, and the immune system.
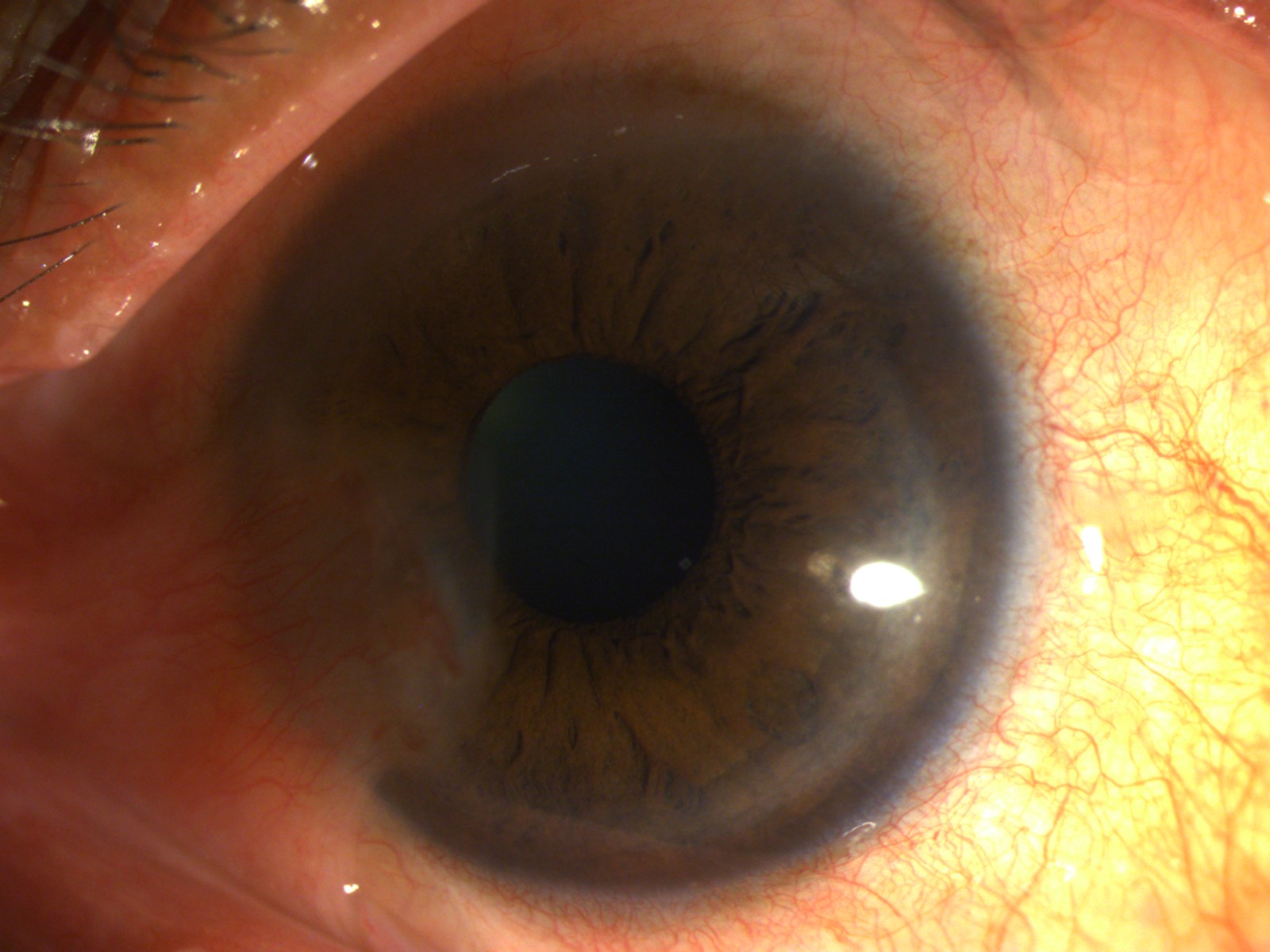
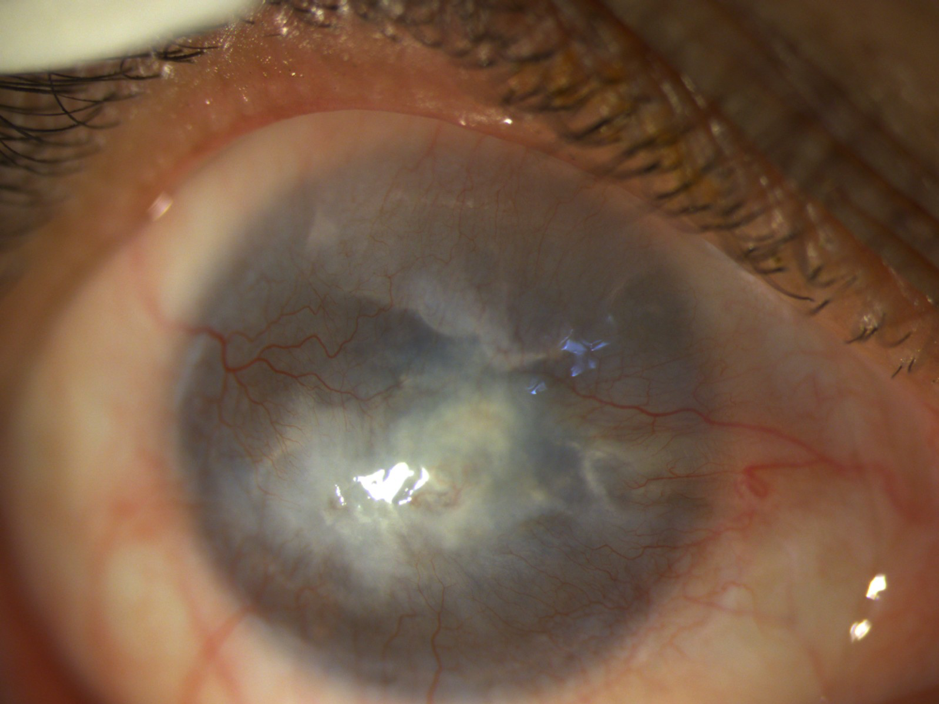
Incomplete conjunctivalisation of the corneal surface is known as partial LSCD (Figures 2, 3) and a complete loss of corneal epithelial stem/progenitor cells leads to total LSCD (Figure 4)with deleterious effects on corneal wound healing and surface integrity.
CLASSIFICATION OF LSCD:
LSCD could be secondary to a known cause or idiopathic [3]. It can be divided into hereditary and acquired types.[Table 1]
|
Hereditary LSCD |
Acquired LSCD |
|
Congenital aniridia |
Chemical injury |
|
Xeroderma pigmentosum |
Thermal injury |
|
Epidermolysis bullosa |
Stevens-Johnson syndrome/toxic epidermal necrolysis |
|
Ectrodactyly-ectodermal dysplasia-clefting syndrome |
Allergic ocular surface disease (Vernal and Atopic keratoconjunctivitis) |
|
Autoimmune polyendocrinopathy-candidiasis–ectodermal dystrophy/dysplasia (multiple endocrine deficiency, APS1) |
Drug-induced LSCD (Mitomycin C, Fluorouracil, Preservatives, Systemic chemotherapy and immunotherapy) |
|
Keratitis ichthyosis deafness syndrome |
Radiation injury |
|
Lacrimo-auriculo-dental-digital syndrome |
Graft-versus-host disease |
|
Dyskeratosis congenita |
Mucous membrane pemphigoid |
|
Contact lens wear |
|
|
Multiple surgeries involving the limbus |
|
|
Infectious keratitis extending to limbus |
|
|
Severe pterygium |
|
|
Severe blepharitis/ rosacea/ trachoma |
|
|
Ocular surface tumors |
Out of these, ocular surface burns are responsible for most of the cases of unilateral LSCD. In bilateral LSCD, the leading identifiable causes are ocular surface burns, allergic conjunctivitis, Stevens-Johnson Syndrome (SJS) or Toxic Epidermal Necrolysis (TEN), aniridia and MMP [5].
CLINICAL FEATURES:
Demography: Male patients outnumber females by about 2:1. Children under 18 years of age comprise almost a third of all cases of LSCD. The percentage of children is higher in cases of unilateral LSCD compared to cases of bilateral LSCD [5].
Symptoms:
Though asymptomatic initially, patients may complain of ocular discomfort, irritation, foreign body sensation, conjunctival redness, tearing, dryness, blepharospasm, recurrent episodes of pain (due to epithelial breakdown), photophobia, decreased vision, and eventually even blindness.
Signs:
The absence of the corneal epithelium phenotype or the presence of conjunctival epithelial cells (conjunctivalization) of the cornea leads to clinical signs of LSCD. In LSCD, the epithelium on the corneal surface is replaced by either conjunctival epithelium with or without neovascularization or a mixture of metaplastic corneal epithelial cells and conjunctival epithelial cells.
On slit-lamp examination, the cornea appears lustreless, and affected areas may have a dull light reflex with or without superficial vascularization, and fibrovascular pannus, recurrent or non-healing epithelial defects, and resultant stromal scarring. Absence or flattening of the limbal Palisades of Vogt may be noted. We can differentiate between corneal and conjunctival epithelium using a fluorescein staining pattern under cobalt blue light. There is a marked difference in the thickness between the corneal epithelium, which tends to be regular and thick, and conjunctival epithelium, which tends to be irregular and thin on the cornea and shows the pooling of fluorescein (as conjunctival epithelium has relatively loose cell-cell contact junctions, which result in a permeability that is up to 40 times greater than that of the corneal epithelium). The pattern and intensity of fluorescein staining vary according to the severity of the disease.
In mild or early disease, which often is sectoral, only punctate fluorescein staining in a curve-like path may be visible in the affected area. As LSCD progresses, there may be vortex keratopathy or whorl-like epitheliopathy and epithelial defects, later on, that may persist and lead to melts and perforations.
STAGING OF LSCD:
The staging of LSCD is important to guide medical and surgical planning. The most important factors include the involvement of the visual axis or central 5 mm of the cornea and the number of clock hours of LSCs affected.
Based on Clinical Presentation: [4] (Figure 5)
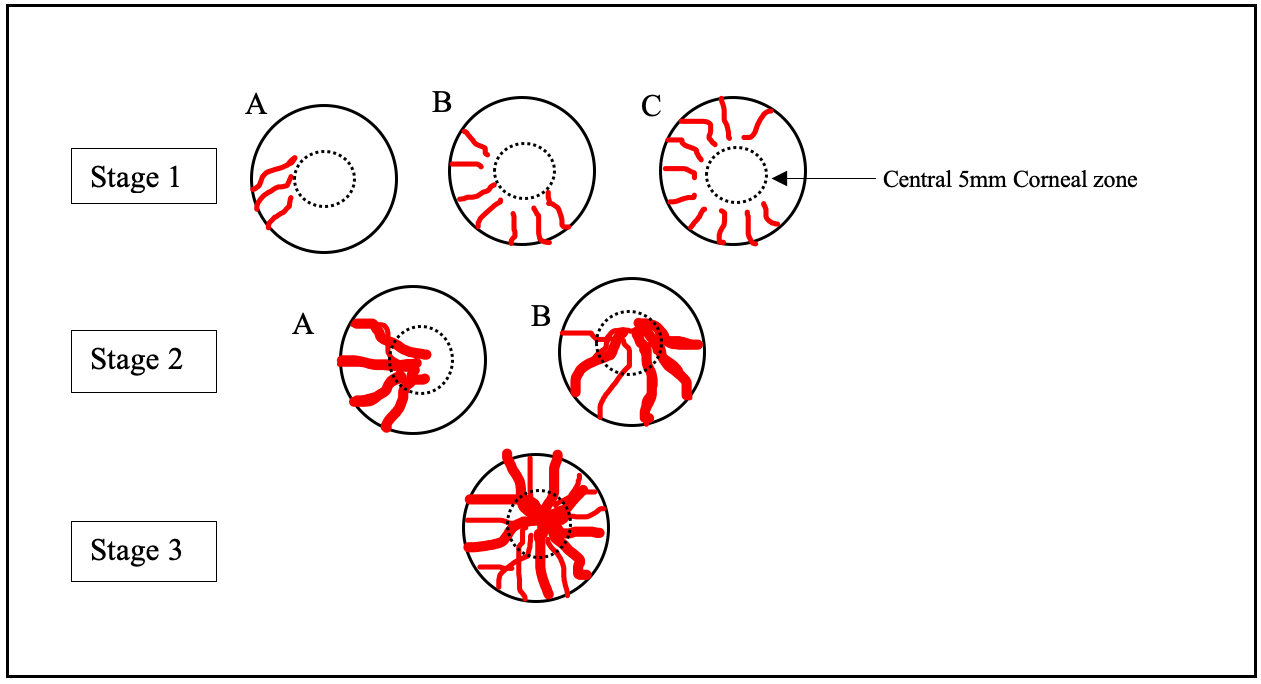
Stage I- Normal corneal epithelium within the central 5 mm zone of the cornea (A: <50% of limbal involvement; B: > 50% but <100% of limbal involvement; C: 100% of limbal involvement) (Figure 3)
Stage II- The central 5 mm zone of the cornea is affected (B: <50% of limbal involvement; B: >50% but <100% of limbal involvement)
Stage III- The entire corneal surface is affected. (Figure 4)
Abnormalities of other components of the ocular surface such as the conjunctiva, meibomian glands, lacrimal glands, tear film, corneal nerves, and immune system are important in the management of LSCD too.
DIAGNOSTIC TESTS:
Though mainly a clinical diagnosis, adjunct diagnostic tests may help in confirming LSCD [4].
- Corneal Cell Sampling
- Phenotypic analysis of ocular surface cells by impression cytology (however, it is not a quantitative test to stage LSCD due to inherent sampling limitations)
- Corneal biopsy to identify conjunctival phenotype. (This is more invasive and a sample is often retrieved during surgical management of LSCD)
- Cell biomarkers
- Histopathological examination (with haematoxylin and eosin or periodic acid-Schiff stain) to detect goblet cells collected by impression cytology or cell scraping to show the presence of goblet cells. However, a negative result cannot rule out LSCD
- Immunohistochemistry for detection of intracellular proteins, eg, cytokeratins (CK) and secreted proteins that are typically expressed in conjunctival epithelial and goblet cells like CK 19, CK7, CK13, MUC1 and MUC5AC.
- Reverse transcription-polymerase chain reaction (RT-PCR) for detecting specific mRNA that is expressed in conjunctival cells collected by impression cytology.
- In Vivo Imaging Techniques
- In vivo laser scanning confocal microscopy (IVCM) can help in grading the severity and monitor any treatment.
|
Feature |
Normal Eye |
LSCD |
|
|
1 |
Corneal basal epithelial cells |
Well-defined, bright cell borders, hypo- reflective cytoplasm, nuclei are not visible and are very faint. |
Cells are absent |
|
2 |
Sub- basal nerve plexus |
Present with normal nerve density |
Sub- basal nerves are fragmented and decreased nerve density |
|
3 |
Palisades of Vogt |
Clearly visible |
Absent/indiscernible |
|
4 |
Goblet cells |
Absent |
May be visible |
|
5 |
Inflammatory cell infiltrates |
Absent |
sometimes visible |
- Anterior segment optical coherence tomography (AS-OCT) may prove useful in measuring epithelial thickness, pannus depth, assessing POV, limbal crypts, and the clear transition between the hypo- reflective corneal epithelium and hyperreflective conjunctival epithelium in the limbal region. It is also important to assess the corneal thickness to plan necessary intervention.
MANAGEMENT OF LIMBAL STEM CELL DEFICIENCY:
Historically, Tseng et al had successfully used amniotic membrane transplantation (AMT) and mechanical debridement and advocated its use to treat patients with partial stem cell deficiency [6].
Acute and semi- acute stage: Preventing further LSC damage and loss of LSC reserve: [7]
Optimization of the ocular surface in the acute and semi acute stages of acquired disorders like ocular surface burns, SJS/TEN, or OCP is necessary to prevent their severe sequelae. This can be accomplished by reduction of surface inflammation, dryness, dealing with neurotrophic issues, disease-specific therapy such as antiallergics or immunomodulators.
Nonhealing epithelial defects in the acute or exacerbatory stage should be addressed appropriately. Amniotic membrane grafting (AMG) in the acute stage of SJS helps in quick healing of the corneal and bulbar conjunctival epithelial defects and specifically the tarsal conjunctival defects thereby preventing the occurrence of lid margin keratinization later.
In acute chemical injuries, ensure adequate lavage and removal or debridement of all of the offending agent and necrotic material. If any limbal or scleral ischemia involving more than 3 to 4 clock hours of the limbus is noted, tenon advancement from the surrounding healthy tissue can be done to re-establish vascularity. Placement of AMG promotes survival of residual LSCs and is the therapy for persistent epithelial defect thereby preventing stromal melts and perforations later. This can be combined with the benefits of a temporary tarsorrhaphy to hasten to heal further. Medical supportive treatment in the form of oral Doxycycline/ Tetracycline, Ascorbic acid, and non-steroidal anti- inflammatory therapy along with topical corticosteroids, cycloplegics, preservative-free lubricants and, if needed, anti-glaucoma treatment is continued.
In the acute stage in eyes with severe chemical burns (Dua’s classification grades 5 and 6/ Roper-Hall classification grade 4), allogenic simple limbal epithelial transplantation (Allo- SLET) may also aid in the resolution of inflammation, faster epithelization, lesser formation of symblepharon and lesser need for tectonic procedures or subsequent optical keratoplasties.
Management in the chronic stage:
The initial management of LSCD with severe disease is to prep the ocular surface prior to the definitive surgical approach. The goals are to stabilise the ocular surface and then rehabilitate the patient visually [7] [8].
The following need to be carefully looked into:
1. Tear Film optimization: A dry ocular surface is not amenable to a successful reconstruction. Hence, restoration of the tear film function is key. Any systemic or topical therapy or its preservative causing injury to the ocular surface needs to be reduced or eliminated. Frequent preservative-free lubricants (with or without osmoprotectants and mucolytics) are prescribed. Permanent punctal occlusion can be achieved by thermal/ Unipolar cautery or surgical closure. The autologousserum is an important adjunctive treatment for severe ocular surface diseases. Lateral or medial tarsorrhaphy can decrease tear evaporation and the area of the exposed ocular surface. In moderate dry eye cases of SJS or OCP, minor salivary gland transplantation (MSGT) has been shown to provide additional lubrication and improvement in the health of the ocular surface. Management of meibomian gland disease with warm compresses and eyelid scrubs, automated thermal pulsation, or intense pulsed light is beneficial.
One form of nonsurgical management of LSCD is the use of therapeutic contact lenses like prosthetic replacement of the ocular surface ecosystem (PROSE). By protecting the unstable conjunctivalized epithelium of the cornea from the direct trauma of the lid wiper effect, tarsal conjunctiva, and lid disease and desiccation, the lenses are able to decrease discomfort and the potential risks for an epithelial defect. However, patients need to be monitored for infectious keratitis.
2. Conjunctival Reconstruction: The conjunctival abnormalities must be treated before LSC transplantation for successful postoperative results. Symblepharon or ankyloblepharon formation can be released and AMG or autologous conjunctival grafting along with symblepharon ring placement and tarsorrhaphy may be added as adjuncts.
For the loss or fore-shortening of fornices, cicatrix lysis combined with AMG or autologous conjunctival grafting may help along with the insertion of a conformer/ symblepharon ring/ silicon sheet implant or intraoperative or postoperative application of mitomycin C to prevent re-adhesion. If autologous conjunctival tissue is unavailable, buccal mucosal grafts can be used for conjunctival reconstruction.
In OCP, severe SJS, and graft-versus-host disease (GVHD), systemic immunosuppression should be initiated to prevent or control exacerbation of the conjunctival cicatrization.
3. Eyelid and adnexal abnormalities: A tailored approach to correct the lid and adnexal abnormalities by concerted efforts from corneal surgeons and ‘Ocular Surface- plasty’ experts is necessary.
Lid margin and surface keratinization and scarring of the tarsal conjunctival plate requires re-placement with oral mucous membrane grafts (MMG).
Lagophthalmos, entropion, ectropion, distichiasis, and trichiasis need correction prior to stem cell therapy or corneal transplantation for optimum results.
Surgical Treatment of Stages 1 and 2A LSCD (Unilateral or Bilateral):
Asymptomatic patients with unilateral or bilateral partial and peripheral conjunctivalisation (Stages 1 and 2A, as proposed by the International LSCD Working group) may not require intervention and medical treatments that optimize the ocular surface as described above are often sufficient. However, if progressive and threatening vision, surgical intervention may be planned.
Sequential sectorial conjunctival epitheliectomy (SSCE) has been mentioned, where the conjunctival epithelium covering a sector of the cornea is excised, one or more times in sequence to prevent it from invading the visual axis until the corneal phenotypic epithelium takes over. But its actual long-term benefits are unproven.
As a temporary measure, AMG can be used to cover the epithelial defect after debridement of the central pannus, allowing for reepithelization.
Surgical Treatment of Stages IIB and III LSCD:
Surgical treatment is often necessary for stages 2B and 3 LSCD. A customized comprehensive treatment for each eye is the best approach to manage such cases. As discussed earlier, aggressive anti-inflammatory therapy using topical and systemic corticosteroids or other anti-inflammatory drugs may reduce irreversible damage to the LSC reserve and niche especially for subacute- and chronic-phase inflammatory disorders, such as in OCP, prior to undertaking a surgical reconstruction.
Further treatment here depends on the:
- Laterality of LSCD- Unilateral or bilateral
- Clarity and thickness of underlying corneal stroma- to plan for initial, simultaneous or subsequent lamellar or penetrating keratoplasty for tectonic and/or optical purposes
- Presence of surface dryness
A dry ocular surface precludes Limbal stem cell transplantation (LSCT) as a non-conducive environment does not support LSC survival.
Unilateral Severe or Total LSCD:
In unilateral severe or total LSCD with a moist ocular surface, where the other eye is healthy, autologous LSCT is preferred over allogenic LSCT due to better graft survival rates and avoidance of systemic immunosuppression.
The different types of Autologous LSCTs are:
1. Conjunctival-limbal autograft (CLAU)- where 2 limbal grafts, each 2 to 3 clock hours and separated at 180 degrees, are harvested from the normal donor eye and transplanted to the affected eye. It is indicated in cases of unilateral total LSCD with severe symblepharon. There is no risk of rejection and the long-term results of this procedure are good. But, there is a risk of Iatrogenic LSCD in the donor eye.
2. Cultivated limbal epithelial transplantation (CLET)- Autologous LSCs are harvested from the normal eye (2 mm× 2 mm) and then cultivated ex vivo over a period of 2 to 3 weeks, and are then transferred to the affected eye as a confluent epithelial sheet. The long-term success rate is 50- 86% and is almost similar to that achieved by CLAU. However, CLET requires a specialized laboratory for culture and is expensive. It is a two-staged procedure.
3. Simple limbal epithelial transplantation (SLET)- This single staged procedure involves the in vivo expansion of autologous LSCs harvested from the contralateral normal eye. A 2mm × 2mm small limbal graft is taken from the donor eye, divided into smaller pieces, and expanded in vivo on a fresh amniotic membrane graft and anchored over it with fibrin glue. The outcomes of SLET are comparable with CLAU and CLET. As smaller donor tissue is used, it reduces the risk of iatrogenic LSCD in a healthy eye. It is cost-effective as it does not need a specialized laboratory for culture and is a single-staged procedure. It is generally performed after the subsidence of the acute inflammatory phase of the disease for best results [9] [10].
In moist eyes with severe or total unilateral LSCD and relatively clear corneal stroma and a normal fornix- a simple Autologous- LSCT (Auto- LSCT) works well. If there is concomitant thinning of the cornea or risk of perforation, then Auto- LSCT is combined with a lamellar keratoplasty (LK) or penetrating keratoplasty (PK) in the same sitting. In the presence of a significantly scarred underlying stroma, Auto- LSCT is later followed by LK/PK after confirming the viability of the posterior segment by either Ultrasound B-Scan or a Diagnostic endoscopy.
For a severely scarred unilateral LSCD in a dry surface, cosmetic procedures are recommended.
Visual Rehabilitation After Successful LSCT:
After the ocular surface is successfully reconstructed, visual rehabilitation can be achieved by scleral contact lenses/ PROSE. If the patient is intolerant to these or if no visual improvement is achieved due to stromal scars, then either optical penetrating keratoplasty or deep anterior lamellar keratoplasty may be considered. Keratoplasty as the primary treatment for LSCD without some form of LSC replenishment is not advisable as the graft will not survive in eyes with severe or total LSCD. Cataract surgery may also lead to better visual outcomes.[11]
Bilateral Severe or Total LSCD:
In bilateral total LSCD with moist eyes and formed fornices, LSCT is performed using allogenic sources (ALLO- LSCT), which could be:
- Conjunctival-limbal allograft from a living-related donor (lr-CLAL)
- Keratolimbal allograft (KLAL) from a cadaveric donor
- Allogeneic Simple limbal epithelial Transplant (ALLO- SLET)
- Cultivated oral mucosal epithelial transplantation (COMET)
Allo- LSCT is done in combination with systemic immunosuppressive therapy to prevent rejection and maintain the ocular surface. Cyclosporine, mycophenolate Mofetil and tacrolimus are the most commonly prescribed immunosuppressive drugs along with systemic steroids. Collaboration with a rheumatologist or an internist is necessary to reduce morbidity and mortality. COMET can be considered to avoid immunosuppression.
In moist eyes with severe or total bilateral LSCD and relatively clear corneal stroma and a normal fornix, Allo- LSCT with immunosuppression works well. With associated thinning of the cornea or risk of perforation, then Allo- LSCT is combined with a lamellar keratoplasty (LK) or penetrating keratoplasty (PK) under immunosuppressive cover. In the presence of a significantly scarred underlying stroma, Allo- LSCT is followed by LK/PK along with concomitant immunosuppression.
Keratoprosthesis (Kpro):
If the Allo- LSCT fails or if immunosuppression is not possible, Type1 Keratoprosthesis (Boston Type1 KPro or the indigenous version, Auro -KPro) can be implanted in well-formed fornices like in cases of aniridia or chemical injury. The postoperative regimen to be followed for the Type 1 KPro is strict and includes long-term use of topical antibiotics (a combination of the fourth-generation fluoroquinolone and vancomycin daily with prophylactic use of topical 1% voriconazole for 5 days once a month), lubricants, tapering doses of topical steroids indefinitely and antiglaucoma medication if required. The BCL is replaced every 2-3 months.
In eyes with severe dryness, severe forniceal shortening or ankyloblepharon, and/or in the presence of severe chemical injury (with severe forniceal shortening and keratinization), SJS, and MMP, Type 2 KPro is preferred, due to the high risk of carrier graft melt.
The choice of a specific Type 2 KPro is based on surgeon preference, patient, and Kpro-related factors. In general, KPro in unilateral LSCD and bilateral KPro implantations are discouraged globally, as the second eye is preferred to be reserved as a spare eye for the future if the need arises or when better implants are available.
If the lids are normal then Modified osteo-odonto-keratoprosthesis (MOOKP), osteo-keratoprosthesis, or Boston Type 2 KPro may be implanted. However, in deficient lids MOOKP, osteo- KPro, and the Lux keratoprosthesis are better options. Adjunctive procedures could be anterior vitrectomy and Ahmed glaucoma valve implantation for IOP control. The proper counseling of KPro patients and their families should emphasize the need for patient compliance with medications and postoperative restrictions and regular follow-ups.
Future Direction and Research:
For bilateral disease, the generation of LSCs from residual LSCs by ex vivo cultivation or from other cell sources including oral mucosa epithelial cells, adipose tissue-derived mesenchymal stem cells, embryonic stem cells, and induced pluripotent stem cells are being studied. Also, small molecules, growth factors, and microRNAs that revive the function of residual LSCs and reconstruct the LSC niche are potential new approaches under research [8]. The availability of a biosynthetic cornea, that when implanted would be repopulated by host stromal cells and nerves, is also in the pipeline and may prove to be an effective substitute for human tissue, wherever necessary.
