Introduction:
The clinical entity of Central Retinal Vein Occlusion (CRVO) has been known since 1878 (1), and it is a common visually disabling disorder that may cause significant ocular morbidity.
It commonly affects men and women equally and occurs predominantly in persons over the age of 65 years (2-4). Associated systemic vascular disease, including hypertension and diabetes, are present in these population groups.
The prevalence rates range from 0.1% to 0.5% in the older adult population. (3-5) Younger individuals may have an underlying hypercoagulable or inflammatory Etiology (6,7) CRVO is the second most common retinal vascular disorder after diabetic retinopathy and is considered to be an important cause of visual loss. (8)
The annual risk of developing any type of retinal vascular occlusion in the fellow eye is approximately 1% per year, and it is estimated that up to 7% of persons with CRVO may develop CRVO in the fellow eye within 5 years of onset in the first eye (9-11).
CRVO is characterized by occlusion of the central retinal vein and consecutive damming of the venous blood flow. Though the exact etiology is poorly understood, it is suspected that venous occlusion induces an ischemic and hypoxic state that leads to visually significant sequelae including macular edema along with anterior segment and retinal neovascularization, leading to profound vision loss as a result of macular edema/ischemia, or in more advanced stages, vitreous hemorrhage, and neovascularization. (12) Anti-VEGF therapy, including ranibizumab and aflibercept, have been shown to be safe and effective in large randomized controlled trials (RCTs) and have been approved by regulatory authorities across the globe. Retinal involvement in CRVO is more extensive than in BRVO and is often accompanied by more severe morphologic and functional impairment (13,14) CRVO may impact a person’s ability to perform activities of daily living, especially in cases of bilateral CRVO.
Pathophysiology
The pathogenesis of CRVO has been a subject of great controversy since its first description by Michel in 1878. (15) The subject is of great importance, not only because of the common occurrence of retinal vascular occlusion and the marked visual disturbances associated with it but also because a proper understanding of its pathogenesis may give a clue to the management of this condition.
Hayreh (16, 17) conducted experimental studies in rhesus monkeys on this subject and showed that:
1. Occlusion of the central retinal vein at its site of exit from the optic nerve is associated with engorged and turgid retinal veins only, and with no other retinal damage.
2. Occlusion of the central retinal artery at its site of entry into the optic nerve immediately produces the classic clinical picture of arterial occlusion. Six to nine days after the occlusion, ophthalmoscopy reveals more- or less normal retinal arteries.
3. Simultaneous occlusion of the central retinal artery and vein at their site of entry and exit from the optic nerve produces the classic clinical picture usually associated with central retinal vein occlusion i.e., retinal hemorrhages, engorged retinal veins, and retinal edema.
Klein et al (18) have postulated three possible mechanisms.
1. Occlusion of the vein by external compression by sclerotic adjacent structures (i.e. central retinal artery and fibrous tissue envelope) and secondary endothelial proliferation;
2. Occlusion by primary venous wall disease(degenerative or inflammatory in nature); and
3. Hemodynamic disturbances produced by a variety of factorslike an arterial spasm, sudden reduction of blood pressure
It was concluded from these findings that arterial ischemia is an important factor in the production of the clinical picture of central retinal vein occlusion. Various local and systemic factors play a role in the pathological closure of vasculature.
Central Retinal Artery (CRA) & Central Retinal Vein (CRV) share a common adventitial sheath, as they exit from the ONH and pass through a narrow opening in lamina cribrosa. Because of this narrow entry in lamina cribrosa, vessels are in a tight compartment with limited space for displacement. This anatomical position predisposes for the formation of thrombus in the CRV by various factors including slowing of the bloodstream, changes in the vessel wall, and changes in the blood together known as the Virchow’s triad (19)
Atherosclerotic changes in the CRA transform the artery into a rigid structure and impinges upon the pliable CRV causing hemodynamic disturbances, endothelial damage, and thrombus formation. This mechanism explains the fact that there will be an associated arterial disease with CRVO as was suggested by Hayreh in his initial studies even though this association hasn’t been proven consistently.
Thrombotic occlusion of CRV can occur as a result of various pathological insults, including compression of the vein (mechanical pressure due to structural changes in lamina cribrosa eg glaucomatous cupping, inflammatory swelling in the optic nerve, orbital disorders), hemodynamic disturbances (associated with hyper or hypo dynamic circulation), vessel wall changes (vasculitis) and changes in the blood (deficiency of thrombolytic factors, increase in clotting factors) (20)
Whatever the mechanism of CRVO, it leads to a backup of blood in the retinal venous system and increased resistance to venous blood flow. This causes stagnation of blood and ischemia of inner retinal layers. This leads to the production of angiogenic factors which stimulate abnormal vascularization of the posterior and anterior segments. Also, there is a breakdown of the inner retinal barrier at the retinal capillary endothelium, leading to abnormal leakage of fluid in the retinal layers causing macular edema.
The prognosis of CRVO depends on the reestablishment of the venous system by recanalization, dissolution of the clot, or formation of optociliary shunt vessels. (21)
In cases with combined arterial and venous occlusion in the retina, the most probable sequence of events is: arterial occlusion (partial or complete, transitory or prolonged) leads to slowing down or stoppage of the circulation in the retinal vascular bed, which, in turn, leads to venous stasis. The venous stasis in a susceptible individual precipitates venous thrombosis. This is further suggested by the fact that most of these episodes come on during sleep when there is systemic arterial hypotension.
Risk factors
Risk factors and associations with central retinal vein occlusion (11,22)
|
Systemic vascular diseases |
Diabetes mellitus, Hypertension, Carotid insufficiency |
|
Ocular diseases |
Open-angle glaucoma, Ischemic optic neuropathy, Pseudotumor cerebri, Tilted optic nerve heads, Optic nerve head drusen |
|
Hematologic alterations |
Hyperviscosity Syndromes: Dysproteinemias (multiple myeloma), Blood dyscrasias : (Polycythemia vera, Lymphoma, Leukemia, Sickle-cell disease or trait), Anemia, Elevated plasma homocysteine Factor XII deficiency, Antiphospholipid antibody syndrome, Activated protein C resistance, Protein C deficiency, Protein S deficiency |
|
Inflammatory/autoimmune vasculitis |
Systemic lupus erythematosus |
|
Medications |
Oral contraceptives, Diuretics, Hepatitis B vaccine |
|
Infectious vasculitis |
HIV, Syphilis, Herpes zoster, Sarcoidosis |
|
Others |
After retrobulbar block, Dehydration Pregnancy |
Clinical features:
The distinctive features of CRVO make the diagnosis easy. In the past when RVO involved half of the retina, it was always considered to be a variant of BRVO but our studies showed that this is generally HCRVO, pathogenetically and clinically a variant of CRVO.
Patients with nonischemic CRVO may have no symptoms and it may be detected as an incidental finding on a routine ophthalmic examination.
Mild retinal hemorrhages areper seasymptomatic. It is almost always the development of macular edema that makes it symptomatic. Blurring of central vision with sparing of peripheral vision is the usual presentation. The onset of symptoms is usually gradual, but it may be discovered suddenly on waking one morning. At least one-third of patients (23) gives a history suggestive of amaurosis fugax especially when linked with combined cilioretinal artery occlusion.
Classification:
Central Retinal Vein Occlusion has been classified by SS Hayreh (24) into :
1: Ischemic (Hemorrhagic retinopathy).
2: Non-Ischemic (Venous stasis retinopathy)
Diagnostic parameters to differentiate the 2 types of CRVO have been described. (23) These tests can be divided into functional tests and morphological tests. Function tests consist of visual acuity, visual fields, relative afferent pupillary defect, and electroretinography. Morphological tests include ophthalmoscopy, fluorescein fundus angiography, and optical coherence tomography.
Functional tests:
Visual Acuity:
Visual acuity of better than 20/200 (6/60) was seen in 58% of the non-ischemic, as compared to only 1.7% in the ischemic type of CRVO. A visual acuity of better than 20/400 (6/120) was seen in 81% of the patients of the non-ischemic, as compared to about 7% of the ischemic type of CRVO only
Peripheral visual fields:
Recorded with a Goldmann perimeter 3 isopters: I-2e, I-4e, and V-4e. It was found that in non-ischemic CRVO, the peripheral visual fields with V-4e and I-4e were normal, and they may even be normal with I-2e in spite of the presence of peripheral visual field defects. In ischemic CRVO, however, the eye may or may not see V-4e, or may or may not see I-4e, and usually does not see the I-2e target at all. Thus, if an eye can see I-2e or has normal visual fields with I-2e, that is in favor of being a non-ischemic CRVO. If an eye can see only V-4e or no target at all, that eye would most probably have ischemic CRVO (25)
Relative afferent pupillary defect:
Measured by photographic neutral density filters it was observed that in non-ischemic CRVO, 97% of the eyes had a relative afferent pupillary defect of <0.6 log units. In contrast to that, in ischemic CRVO, 94% of the eyes had a relative afferent pupillary defect of >0.9 log units and 91% of the eyes >1.2 log units
Electroretinography:
The most sensitivity and specificity test to differentiate ischemic from non-ischemic CRVO is amplitude of the b-wave (in both photopic and scotopic ERG). If the amplitude of b-wave is <60% of the normal, then there is an 80% chance that we are dealing with an ischemic CRVO. Or, if the amplitude of the b-wave is reduced by one or more standard deviation of the equipment, then we have a high chance of that being an ischemic CRVO.
Morphological tests:
Ophthalmoscopy:
It is usually felt that extensive retinal hemorrhages and cotton-wool spots suggest an ischemic CRVO though this is not considered a very reliable sign to differentiate the two forms of CRVO as appearances can be similar in ischemic and non-ischemic CRVO, during both early and late stages of the disease, and can be misleading.
Fluorescein Angiography:
Typically retinal capillary non-perfusion is considered a diagnostic criterion of ischemic CRVO, but in view of extensive retinal hemorrhages, media opacity (cataracts / small pupil), fluorescein fundus angiography provides information on retinal capillary non-perfusion in only 2/3 of the eyes during the acute phase.
The CVOS classified the perfusion status of a CRVO as perfused, nonperfused, or indeterminate based on fluorescein angiographic characteristics.
A multicentre study trial showed that 10 Disc areas of retinal capillary non-perfusion is not an ideal criterion for diagnosing ischemic vs non-ischemic CRVO. The study has shown that eyes with less than 30-disc diameters of retinal capillary nonperfusion and no other risk factor are at low risk for developing iris/angle NV, whereas eyes with 75 disc diameters or more are at the highest risk (23).
RAPD and ERG changes information, provides the most reliable way of differentiating the two types of CRVO in as many as 97% of the cases(23 )
Diagnosis:
Retinal vascular disease is the most common retinal vascular disorder after diabetic retinopathy. Early recognition and treatment are important to avoid potential significant visual morbidity. The diagnosis of CRVO is not difficult, but differentiation of non-ischemic from ischemic CRVO needs more detailed analysis. This differentiation is important for the correct management especially for prognostication and follow-up of CRVO because non-ischemic CRVO is a moderately benign condition but ischemic CRVO is often associated with serious blinding complications.
History
An initial history should consider the following elements:
- The duration of vision loss
- Current medication
- Systemic history (e.g., systemic hypertension, diabetes, hyperlipidemia, cardiovascular disease, sleep apnea, coagulopathies, thrombotic disorders, pulmonary embolus)
- Ocular history (e.g., glaucoma, other ophthalmologic disorders, ocular injections, surgery, including retinal laser treatment.
Ophthalmic Examination
The initial examination should include the following elements.
- Visual acuity
- IOP
- Pupillary assessment for a relative afferent pupillary defect corresponds to the level of ischemia.
- Slit-lamp biomicroscopy, looking carefully for fine, abnormal new iris vessels (NVI)
- Gonioscopy for angle neovascularization (ANV) prior to dilation, especially in cases of an ischemic CRVO, when there is an elevated IOP or when iris neovascularization risk is high.
Binocular funduscopic evaluation of the posterior pole.
A dilated examination is preferred to ensure an optimal view of the entire retina and to assess for the following features that often lead to visual impairment:
- Macular edema, detected both clinically and/or by using optical coherence tomography (OCT) imaging
- Signs of ischemia, including neovascularization of the disc or elsewhere, extensive hemorrhages, venous dilation and tortuosity, and cotton wool spots
- Optic nerve head neovascularization and/or neovascularization elsewhere
- Vitreous or preretinal hemorrhage
Ocular Diagnostic Tests
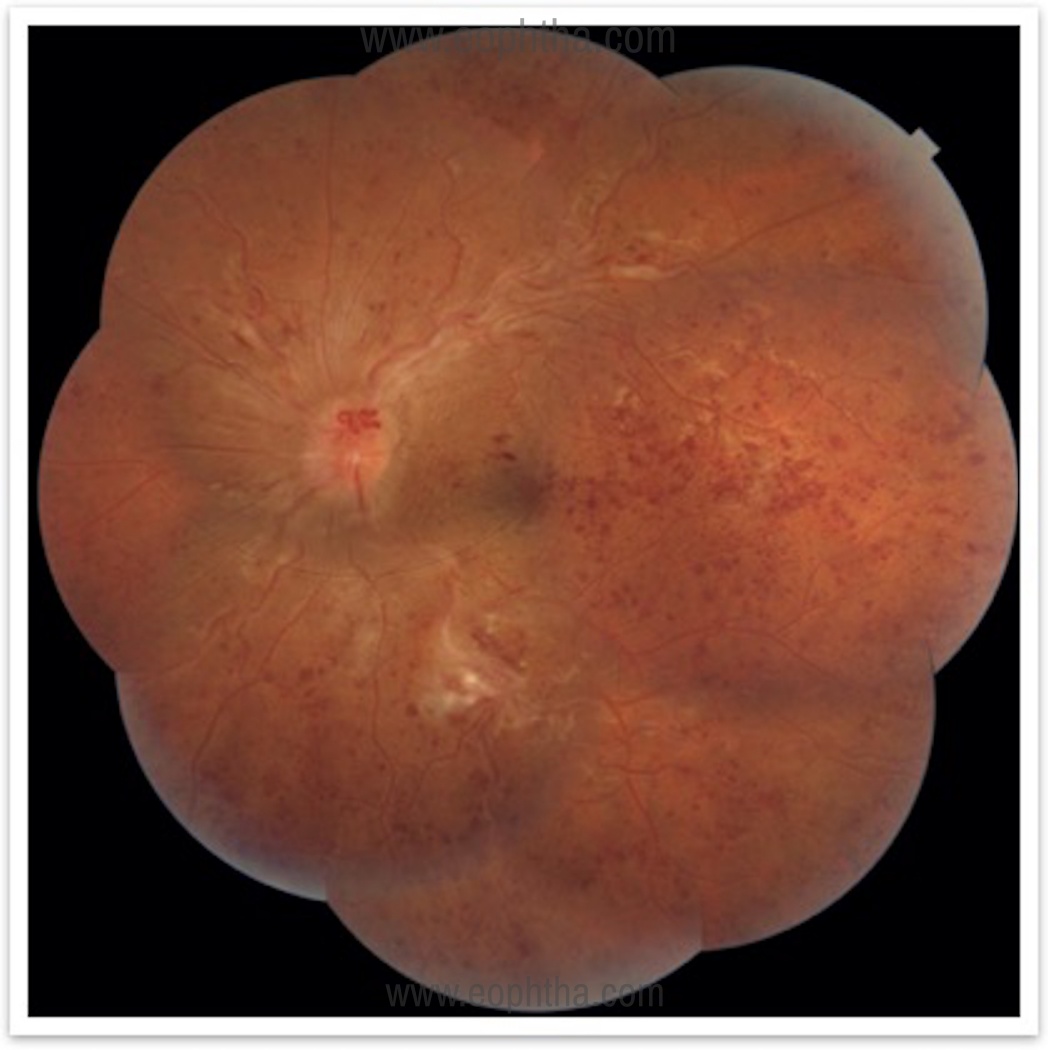
Color fundus photography:
It is useful for documenting the severity of the retinal findings, the presence of new vessels elsewhere in the retina (NVE), or near the optic disc (NVD), the extent of intraretinal hemorrhages. It helps in following up on cases and documenting the response to treatment. (Figure 1)
Optical coherence tomography (OCT)
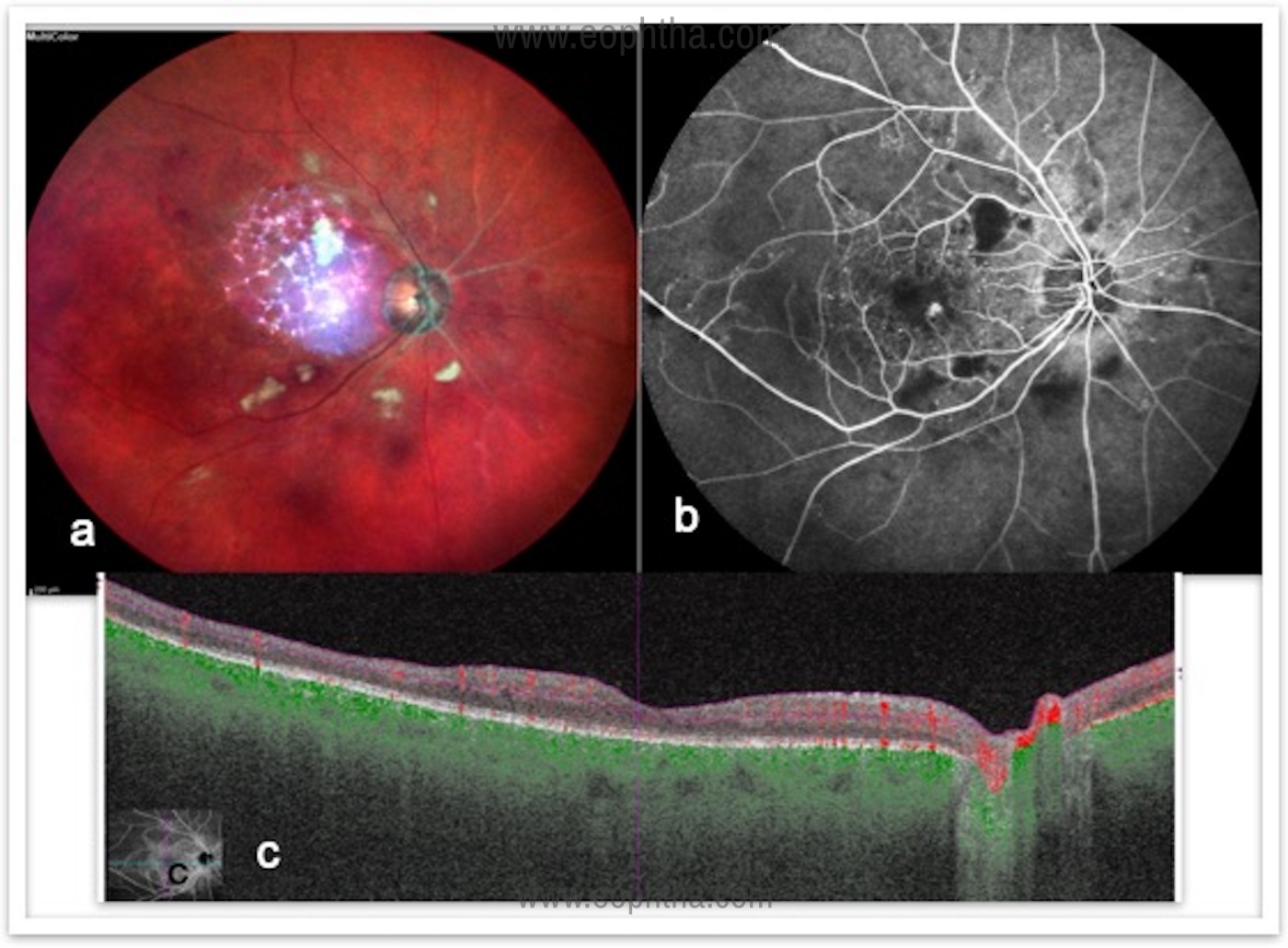
OCT is useful to confirm and quantify the severity of macular edema, assess the integrity of the ellipsoid zone/photoreceptor layers, vitreo-retinal interface changes, subretinal fluid, and monitor response to treatment. In clinical practice, OCT measurements often guide treatment decisions. Large clinical trials testing anti-VEGF treatment are based largely on using quantifiable OCT measurements.
Fluorescein angiography (FA):
Fluorescein angiography is used to evaluate the extent of the vascular occlusion, the degree of ischemia and the type of macular edema (ischemic vs. nonischemic). It enables to localize leaking micro aneurysms or areas of capillary dropout, distinguish collateral vessels. Therefore, helps in treatment decisions and monitoring. (Figure 2)
Wide-field Fluorescein angiography:
A modern technology that is being used to evaluate peripheral nonperfusion, peripheral neovascularization, skip areas post photocoagulation. It helps in the targeted approach in treatment. (Figure 3)
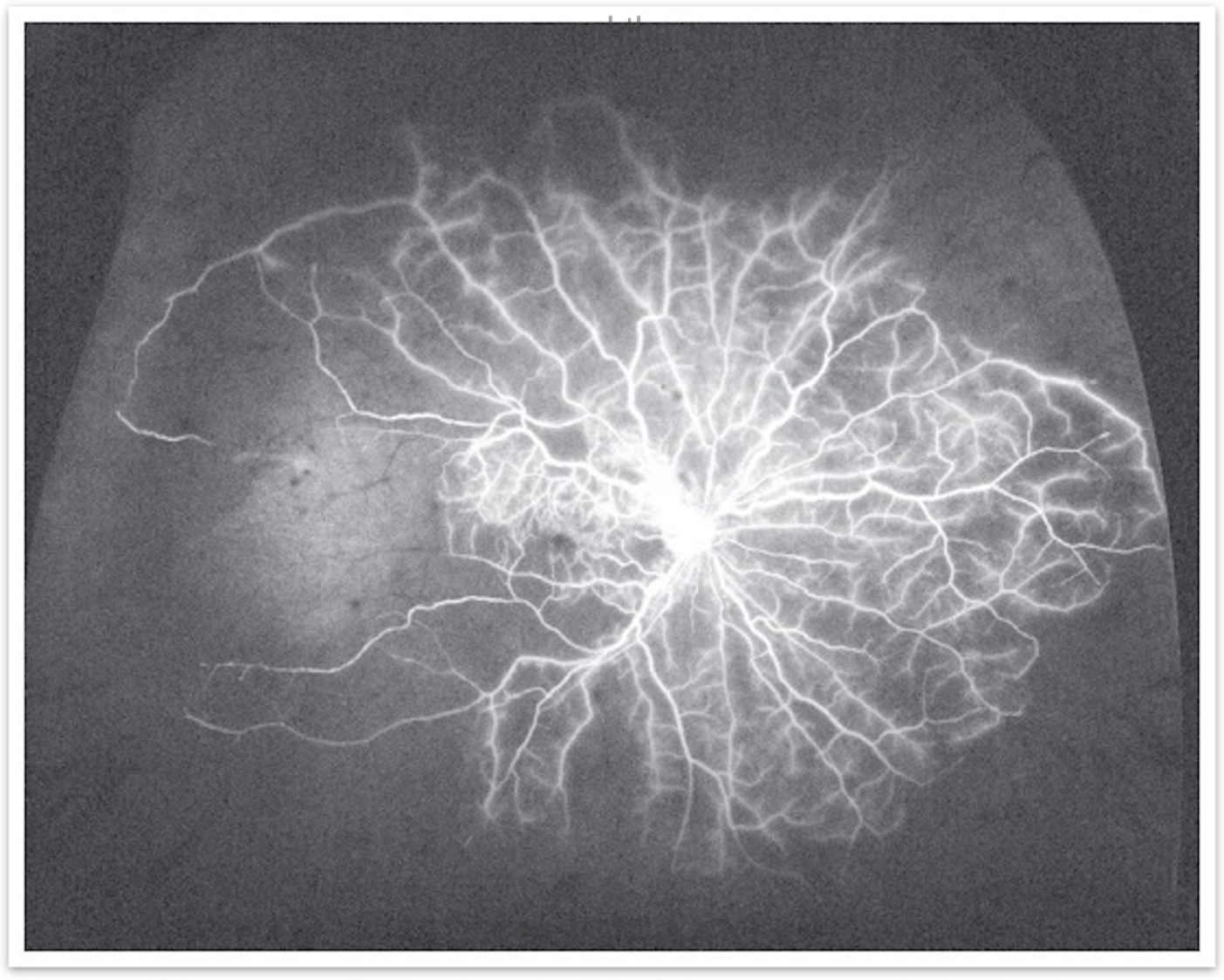
With traditional FFA peripheral areas of retinal non-perfusion may be skipped and those untreated areas may be the source of production of mediators that promote neovascularization and macular edema.
OCT angiography:
This is a novel technique that is dyeless and non-invasive. Excellent for patients with deranged kidney function tests. It can be repeated frequently to monitor response to therapy. Unlike standard fluorescein angiography, OCTA provides details of superficial and deep retinal capillary plexus (Figure 4) and choroidal capillary structures, which are affected by retinal vein occlusions. A Widefield peripheral view can be accessible with the montage view. (Figure 5)
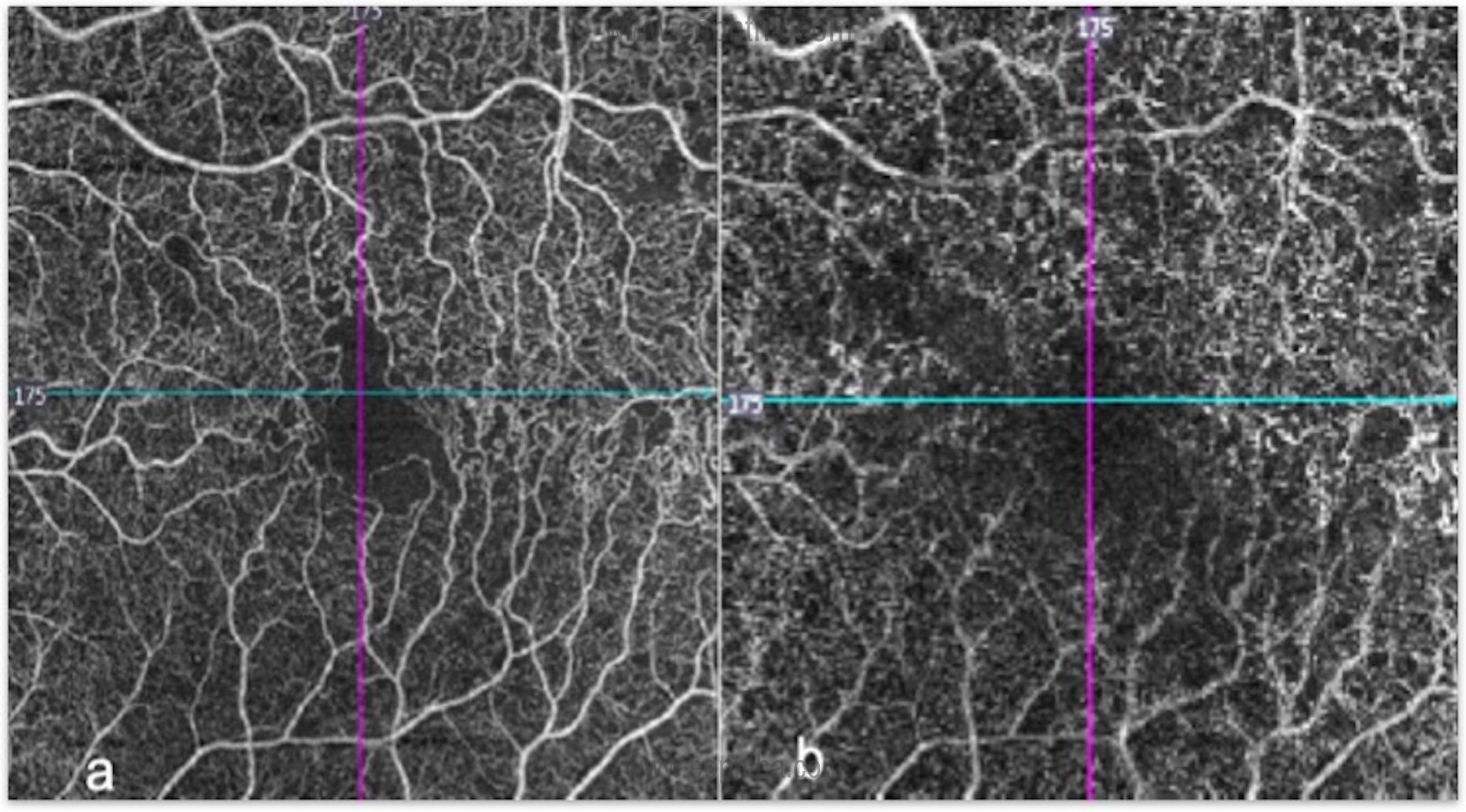
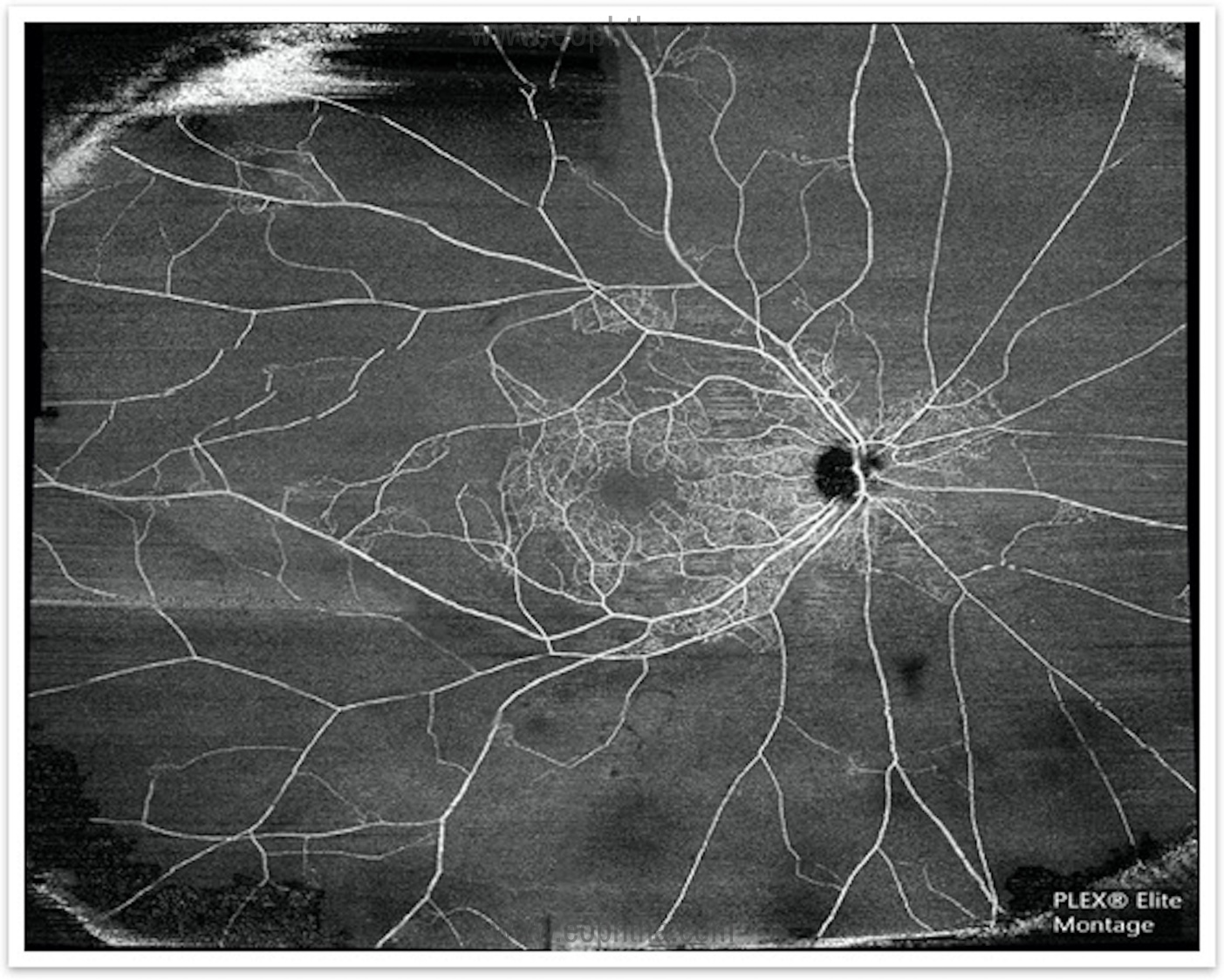
Changes in OCTA of Eyes with RVO:
The changes visible in OCTA can be described as qualitative and quantitative:
|
Qualitative changes |
Quantitative changes |
|
Non-perfusion areas |
Foveal & perifoveal vascular density |
|
Vascular tortuosity |
Non-perfusion area measurement |
|
Collateral vessel formation |
Foveal avascular zone size |
|
NVE/NVD |
|
|
Cystoid spaces |
Ultrasonography:
Ultrasonography is an extremely valuable diagnostic tool that enables the assessment of the anatomic status of the retina in the presence of a vitreous hemorrhage or other media opacity. Ultrasonography is not commonly used with clear media and OCT is more appropriate.
Electroretinography (ERG):
This is also an objective functional test, very useful in the differentiation of ischemic from non-ischemic CRVO. The perfusion of the inner retina is affected, so the amplitude of the b-wave is decreased relative to the a-wave; the b-to-a ratio has been shown to be reduced.
Systemic evaluation of a patient with CRVO:
Patients with more than 60 years of age with a known systemic vascular risk factor do not need a systemic workup, whereas young patients and those with bilateral simultaneous vascular occlusions need a thorough evaluation for hypercoagulable conditions as they can have an increased risk of future non-ocular thrombotic events. (Figure 6)
- Standard Investigations: CBC including ESR, Kidney function tests & Liver function tests, Random Glucose, Lipid profile, Urine analysis
- Thrombophillia screen (< 50 years): Coagulation profile (BT/ CT/ PT/ APTT), Plasma Homocysteine level, Antiphospholipid antibodies, Antinuclear antibodies, Protein C, S deficiency
Treatment:
Treatment of CRVO involves management of the systemic predisposing risk factors, such as diabetes and hypertension along with the ocular condition. The most common causes of the significant drop in vision in CRVO patients are macular edema and neovascularisation-related complications.
The available treatment options for CRVO includes anti-VEGF therapy, intravitreal injection of steroids, Pan retinal photocoagulation, intravitreal injection of tissue plasminogen activator (tPA), and pars plana vitrectomy. Eliminating macular edema (CME) to improve vision, prevention/ treatment of retinal neovascularization, and anterior segment neovascularization & prevention of neovascular glaucoma are important steps.
1. Macular edema:
The gold standard treatment of choice is Intravitreal AntiVEGF injections:
Currently, there are three anti-VEGF drugs are available. Both Ranibizumab and Aflibercept are US FDA approved, while bevacizumab is an off-label use of an anti-VEGF drug. Anti-VEGF therapy is now the treatment of choice for CRVO in preventing vision-threatening problems.
AntiVEGF Trials:
The CRUISEstudy (26), published in 2010, evaluated the effects of ranibizumab (Lucentis, Genentech) in patients with macular edema secondary to CRVO. In the study, patients were randomly assigned to receive monthly injections of 0.3 mg or 0.5 mg ranibizumab or sham injections for 6 consecutive months. At the sixth month, patients were followed monthly and reinjected as needed.In the first 6 months a rapid improvement in BCVA (+18.3 letters for the 0.5 mg group, +16.6 letters for the 0.3 mg group, and +7.3 letters for the sham group;P <.05) that was statistically significant, was observed. It was noted that, at month 12, both ranibizumab groups maintained the visual acuity gains of the first 6 months.
Patients who completed the 12-month CRUISE trial entered an open-label, single-arm, multicentre follow-up study called the HORIZON extension study (27) in which they could continue to receive 0.5mg ranibizumab on a PRN basis. The key finding from the HORIZON was that long-term use of ranibizumab was well-tolerated. CME not treated in the second year was also associated with worse visual and anatomical outcomes. There are clear differences in outcomes for BRVO and CRVO patients. CRVO patients required frequent follow-up and continued ranibizumab therapy to control edema
The RETAIN Study (28) included patients with CRVO and BRVO in a prospective follow-up of a subset of patients from two phases three trials of ranibizumab in RVO. The mean follow-up was 49.7 months for CRVO patients, where 14 of 32 patients (44%) had edema resolution, with 71% receiving their last injection within two years of treatment initiation. However, in unresolved patients, a mean number of 5.9 injections of ranibizumab were given in year 4.
The CRYSTAL study (29) long-term trials support the safety and efficacy of intravitreal ranibizumab for the treatment of CRVO with dosing on an as-needed basis. Patients with CRVO received 0.5 mg ranibizumab, monthly injections until visual acuity was stable for 3 consecutive months, and they were followed to 12 months thereafter, on PRN, basis. The mean change in BCVA at month 12 was 12.3 letters after a mean of 8.1 injections.
COPERNICUS study (30) was a phase three, prospective, randomized, double-masked trial (n=187) comparing monthly intravitreal injection of aflibercept 2mg (n=115) with sham (n=74) for the treatment of CME secondary to CRVO.
This study also showed that unless treatment is instigated early, there is likely to be a great degree of irrecoverable visual loss. The aflibercept group demonstrated a rapid reduction in CRT or CFT by week 24 and was maintained to week 52. Progression to ocular neovascularization during the first 52 weeks was eliminated in the aflibercept group (0% vs. 6.8% in the sham treatment group P=0.006). All neovascularization seen in the sham group occurred in the anterior segment. The results also suggest that once disease control was obtained over several months of loading, less frequent injections were needed subsequently.
GALILEO study (31) was a phase 3, randomized, double-masked trial comparing intravitreal aflibercept with sham for CME (macular edema) secondary to CRVO. There was no crossover in this study. The visual and anatomic improvements seen after fixed, monthly dosing at week 24 were largely maintained when treatment intervals were extended. Patients with macular edema following CRVO benefited from early treatment with intravitreal aflibercept.
A Cochrane meta-analysis (32) on anti-VEGF agents (aflibercept, bevacizumab, ranibizumab or pegaptanib sodium) for the treatment of CME (MO) secondary to CRVO included high–quality data from 937 participants in six RCTs, who were either treated with intravitreal anti-VEGF or sham injection. They found that participants receiving anti-VEGF treatment were 2.7 times more likely to gain at least 15 letters of visual acuity at six months compared to participants with sham. In addition, high-quality evidence from six trials suggested that anti-VEGF treatment was associated with an 82% lower risk of developing iris neovascularization at six months compared to sham injection (RR 0.18; 95% CI 0.09 to 0.36).
Intravitreal steroids
Intravitreal corticosteroids are a treatment option for CRVO. Available options are IVTA (intravitreal triamcinolone acetonide) and dexamethasone implant injection.
The “Standard Care versus Corticosteroid for Retinal Vein Occlusion” (33) trial was a randomized clinical trial that treated individuals with nonischemic CRVO macular edema with 1 mg and 4 mg intravitreal triamcinolone injections every 4 months compared to observation alone. At 12 months, those eyes treated with either dose of triamcinolone were five times more likely to have a 15-letter gain in visual acuity compared to the control group.
Dexamethasone, in its free form, has a short half-life, which limits its clinical usefulness in its naïve form. This was circumvented by using a biodegradable pre-filled applicator which has the property for sustained release. This implant containing 0.7mg of dexamethasone (Ozurdex, Allergan) was analysed in the GENEVA study (34) which showed that the percentage of eyes with ≥ 15 letter gain in BCVA was significantly higher in both implant (0.35 mg/ 0.7 mg) groups compared with sham at days 30 to 90 with a peak effect at 60 days. In terms of safety, raised IOP peaked at month two (3.2% of patients had an IOP>35 mmHg), but declined significantly by month three and was close to 0% by month six, with 19% of patients requiring an IOP-lowering agent at month six and 0.7% of patients requiring any IOP-lowering surgical procedures. Similarly, rates of cataract progression were low with 7% progression at month six, compared to 4% in the sham group.
Laser photocoagulation
Now a day, there is a limited role for grid photocoagulation to treat macular edema. The Central Vein Occlusion Study Group (CVOS) (35) showed that although macular edema reduced, but no improvement in visual acuity is seen after grid treatment and no difference in final visual outcome compared to control eyes.
2. Neovascularisation :
This is a vision-threatening complication following ischemic CRVO. Overall incidence is 45% among the ischemic CRVO eyes.In CVOS (36) eyes, 35% of eyes initially characterized as non-perfused or indeterminate developed NVI/NVA compared to 10% of those initially categorized as perfused.
Salient points:
- Every eye with ischemic CRVO does not develop ocular NV.
- When ocular NV develops, the commonest site is the anterior segment, much less frequently the posterior segment.
- The greatest risk of developing anterior segment NV is during the first 7 months, after which the risk falls dramatically to minimal.
Treatment is aimed at preventing vitreous haemorrhage and neovascular glaucoma. Pan retinal photocoagulation is the gold standard procedure. In this process, laser shots are delivered adequately to cover the ischemic retina to ensure regression of NVI/ NVA over time. Either single shot or multi-spot lasers may be used. Widefield fluorescein angiography / OCT angiography montage is a useful modality in assessing the non-perfusion areas in the peripheral retina and one can have a targeted approach.
Stepwise approach for CRVO
- A systemic approach for risk factors (to be managed by the patient’s physician).
- Ophthalmic management
1. Non-Ischemic CRVO:
Baseline approach: Diagnosis made on multimodal imaging with documented visual acuity.
To document macular edema, OCT is the most commonly used imaging modality. The fact that CRYSTAL (36) resulted in a statistically significant BCVA gain in a broad population of patients, including those with macular ischemia at baseline, supports the notion that focal ischemia or atrophy may not be associated with worse VA.
Which agent to choose?
Although any of these drugs may be used as the first-line for this condition, anti-VEGF is preferred in eyes with a history of glaucoma (diagnosed/suspected) and younger patients who have clear crystalline lens. Dexamethasone implant (Ozurdex) is a better choice in patients with recent cardiovascular events and in those who do not favor monthly injections.
Ranibizumab / Bevacizumab/ aflibercept are the anti-VEGF agents recommended by most of the studies for the treatment of CME due to CRVO. There is no visual acuity or central macular thickness restriction in the commencement of treatment with any of these agents.
Duration of macular edema and visual acuity:
Most participants in the CRUISE trial and Aflibercept trials had less than 3 months’ duration of macular edema. It indicates visual outcome is better with a shorter duration of macular edema. Therefore, early and prompt treatment is of utmost importance. The change in visual acuity after 3 loading doses should help to decide whether further anti-VEGF treatment is worthwhile. Careful consideration should be given to further therapy in eyes that do not improve in terms of visual acuity or macular edema after loading doses. Multiple factors like macular ischemia, structural damage at the fovea, and other confounding factors should be taken into account in the continuation of therapy.
Retreatment and switching agents:
At each follow-up visit, visual acuity, macular thickness and IOP should be assessed and the presence of NVI/NVA assessed. There is a good rationale to switch from dexamethasone implant to an anti-VEGF agent and vice versa as the different modes of actions of these agents may aid in the resolution of edema. After loading doses with either of the intravitreal drugs FFA / OCTA can be performed to identify NV with extensive CNP areas or focal leakages around the macula, and accordingly consider for pan laser photocoagulation can be done to prong the effect of the intravitreal injections.
2. Ischemic CRVO
Patients with ischaemic CRVO are at risk of NVG for which laser photocoagulation is beneficial. The risk of iris neovascularisation is higher if the area of retinal ischemia is >10DD. Pan retinal photocoagulation (PRP) remains the mainstay of treatment when NVI/NVA is visible.
Monitoring:
Ischemic CRVO should ideally be monitored monthly for new vessels /NVI/NVA. PRP is advocated at the earliest sign of iris or angle new vessels. In circumstances when regular follow-up is not possible/impractical, prophylactic treatment is appropriate (37).
Adjunctive agents with PRP:
Anti VEGF agents are used as adjuvants to PRP in patients with anterior segment neovascularisation secondary to ischemic CRVO.
Management of established Neovascular Glaucoma (NVG) :
NVG due to CRVO is basically due to the excessive amounts of VEGF being secreted by the ischemic retina. The management has two main components. The foremost treatment plan is to control the IOP and the other component which is most critical for long term outcome is reduction of the ischemic drive that induces formation of new blood vessels. The neovascularization process needs to be interrupted by injecting anti-VEGF agents like Bevacizumab (Avastin) or Ranibizumab (Lucentis/Accentrix) followed by laser photocoagulation or anterior retinal cryotherapy depending on the media clarity.
If NVI/NVA is present and after gonioscopy anterior chamber angle is open PRP with AntiVEGF agent is recommended.
In conditions where angles are closed and raised intra ocular pressure and if the eye has good visual potential urgent PRP wth cycloablative procedure can be considered or in addition, anti VEGF agent can be considered which will help in regression of new vessels.
In eyes with poor visualization due to cataract / vitreous hemorrhage non resolving with good visual potential one should consider for cataract surgery with pars plana vitrectomy with adjunctive treatment of AntiVEGF should be planned.
DLCP another modality has a definite role in the management of these situations and helps buy time for any definitive intervention. The key to using this effectively and to reduce complications is to adopt a staged approach. Ensure that the patient is appropriately counselled on the risks and outcome. DLCP should only be done once the cause of retinal ischemia has been treated.
Recommendations for follow up:
In eyes with significant ischemia follow up to after six months should be every 3 months for one year. In non-ischemic eyes follow up every 3 months till six months is advised. Subsequent treatment depends upon generally required after two years in uncomplicated cases. The development of disc collaterals/ resolution of CRVO indicates a good outcome.
Surgical Treatment:
Several surgical treatments have been attempted, but none has undergone strict scrutiny in randomizedclinical trials.
Radial optic neurotomy.In this approach, an incision is made in the optic nerve and adjacent retina with a micro vitreoretinal (MVR) blade during pars plana vitrectomy (38). The original idea was to reduce the congestion of the optic nerve by opening the scleral canal, although it has also been hypothesized that this procedure may allow the formation of a retinal-choroidal anastomosis. There have been mixed results of its safety and efficacyand this procedure has fallen out of favor and is rarely performed (39).
Vitrectomy:
Rationale: It is evidence that vitrectomy increases the transport of oxygen to ischemic areas and increases the clearance of VEGF and cytokines in the vitreous cavity. Moreover, the increase in oxygenation reduces VEGF, with a temporary reduction of macular edema. Eyes with non-clearing vitreous hemorrhage from secondary retinal neovascularization in these eyes may require surgical evacuation. At the time of vitrectomy, clearing of the hemorrhage can be combined with removal of epiretinal membranes and fibrovascular proliferations and followed by placement of complete PRP (40). The use of vitrectomy with or without membrane peeling in the management of macular edema secondary to CRVO requires further randomized trials to establish its efficacy compared with the actual gold standard management, which is the intravitreal injection of anti-VEGF drugs.
Tissue plasminogen activator.
Tissue plasminogen activator (tPA) has also been used, both intravitreally as well as through direct injection into a cannulated retinal vein. This approach has not been compared with medical management in a comparative trial (41).
Tackling treatment-refractory CRVO
There have been few recent advances in the treatment of CRVO. Optical coherence angiography has given us an opportunity to study the superficial and deep capillary plexus in patients with retinal vascular diseases (42). The degree of perifoveal capillary non-perfusion has been correlated with visual function.
The degree of retinal non-perfusion may be prognostic for the chance of developing new vessels. The odds ofdeveloping neovascularization go from 0% with less than one-disc area (DA) of non-perfusion to an 80% risk with 75 to 150 DA of non-perfusion(43). The use of wide-field fluorescein angiography has allowed us to better determine capillary non-perfusion and the risk of developing neovascular complications.
RVO-associated macular edema may be refractory to treatment with an anti-VEGF agent.
- Risk factors: suboptimal response include older age, shorter occlusion distance from the optic nerve, longer pre-treatment duration, and larger areas of non-perfusion.
A combination of an anti-VEGF and a corticosteroid drug for the treatment of RVO has been advocated, but there is a paucity of long-term studies supporting this approach. A study by Singeret al (44). showed that combination therapy with an anti-VEGF agent and dexamethasone implant led to a mean re-injection interval of 135 ± 36.4 days for patients with macular edema secondary to CRVO and BRVO as well asimprovements in visual acuity and central foveal thickness.Unfortunately, there was no control group. However, combination therapy remains a possibility for difficult-to-treat eyes.
Future trends:
There is hope that new anti-VEGF drugs will be coming to market in the near future like conbercept and Brolucizumab.
Clearside Biomedical Inc. (Alpharetta, GA, USA) has introduced a novel approach to the treatment of RVOs, using a combination of a suprachoroidal delivery system for the delivery of corticosteroids and intravitrealAflibercept, to improve vision and decrease the treatment burden. In the TANZANITE study (45), 46 treatment-naïve patients with RVO received intravitreal Aflibercept alone or the combination of Aflibercept and concomitant suprachoroidal delivery of triamcinolone acetonide. At three months, the combination arm showed an increase in visual acuity and improved OCT compared with the Aflibercept-alone cohort.
Nanoparticles, liposomes, and other drug delivery systems hopefully will allow less-frequent injections of anti-VEGF agents or corticosteroids.
Conclusion:
In conclusion, RVOs continue to be a commonly encountered retinal condition. With the advent of improved therapies, we now have the ability to treat thesecondary complications of neovascularization and macular edema.
References:
1.Michel J. Ueber die anatomischen Ursachen von Veranderun- gen des Augenhintergrundes bei einigen Allgemeinerkrankun- gen. Deutsch Arch Klin Med 1878;22:339–345) 2. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol 1994;117:429–41. 3 Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol 1996;114:1243–7. 4. The Central Vein Occlusion Study. Baseline and early natural history report. Arch Ophthalmol 1993;111:1087–95. 5. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. 6. Gutman FA. Evaluation of a patient with central retinal vein occlusion. Ophthalmology 1983;90:481–3. 7. Fong AC, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol 1993 ;37:393–417. 8.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc 2000;98:133–141. 9. The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol 1997;115:486–91. 10. Deramo VA, Cox TA, Syed AB, et al. Vision-related quality of life in people 10. Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlu- sion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:313–9 e1 11. The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Arch Ophthalmol 1996;114:545–54. 12. Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther 2008; 16(4): 791–799. 13. Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retina Eye Res. 2014; 41: 1–25. 14. Campochiaro PA, Sophie R, Pearlman J, Brown DM, Boyer DS, Heier JS, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 2014; 121: 209–219. 15. Michel, J.: Die spontane Thrombose der Vena centralis des Opticus. von Graefe's Arch. Ophth. 24:37, 1878. 16. Hayreh, S. S.: An experimental study of the central retinal vein occlusion. Tr. Ophth. Soc. U.K. 84:586, 1964. 17. Occlusion of the central retinal vessels. Brit. J. Ophth. 49:626, 1965. 18. Klien BA. Sidelights on retinal venous occlusion. Am J Ophthalmol. 61:25-35, 1966. 19. Virchow: Cited in A Textbook of Pathology, 7th ed. London, Kimpton, 1961, p. 130. 20. Green WR, Chan CC, Hutchins GM, et al. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Retina 1981;1: 27–55. 21. The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol 1997;115:486–91. 22. Yap YC, Barampouti F. Central retinal vein occlusion secondary to protein S deficiency. Ann Ophthalmol (Skokie) 2007;39:343–4 23. Hayreh SS, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008 Apr;28(4):581-94. 24. Hayreh SS: Classification of central retinal vein occlusion. Ophthalmology 1983;90:458-74. 25. Hayreh SS. Retinal vein occlusion. Indian J Ophthalmol 1994;42:109-32 26. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study.Ophthalmology.2010;117(6):1124-1133. 27.Heler JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: longterm follow-up in the HORIZON trial.Ophthalmology.2012;119(4):802-809. 28.Campochiaro PA, Sophie R, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology.2014; 121:209-129. 29.www.clinicaltrials.gov/ct2/show/NCT01535261 30.Brown DM, Heier JS, and Clark WL et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013; 155(3):429-437. 31.Holz FG, Roider J, and Ogura Y et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol 2013; 97:278–8. 32.Braithwaite T, Nanji AA, Lindsley K, Greenberg PB. Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2014; 5: CD007325. 33.The SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) Study Report 5. Arch Ophthalmol. 2009; 127:1101–1114. 34. Haller JA, Bandello F, Belfort R Jr, et al. Ozurdex GENEVA Study Group; Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011; 118:2453–60 35. Anon. Arandomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion: the Central Vein Occlusion Study Group. Ophthalmology 1995; 102: 1434–44. 36. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995 Oct; 102(10):1434–44 36.Larsen M, Waldstein SM, Boscia F, Gerding H, Monés J, Tadayoni R, et al.; CRYSTAL Study Group. Individualized ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion: twelve-month results of the crystal study. Ophthalmology. 2016 May;123(5):1101–11 37.Hayreh SS, Klugman MR, Podhajsky P, Servais GE, Perkins ES. Argon laser panretinal photocoagulation in ischemic central retinal vein occlusion. A 10-year prospective study. Graefes Arch Klin Exp Ophthalmol. 1990; 228(4):281–96. 38. Opremcak EM, Bruce RA, Lomeo MD, et al. :Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases.Retina.2001;21(5):408–15. 10.1097/00006982-200110000-00002 39.Weizer JS, Stinnett SS, Fekrat S:Radial optic neurotomy as treatment for central retinal vein occlusion.Am J Ophthalmol.2003;136(5):814–9. 10.1016/S0002-9394(03)00698-6 40. Lam HD, Blumenkranz MS. Treatment of central retinal vein occlusion by vitrectomy with lysis of vitreopapillary and epipapillary adhesions, subretinal peripapillary tissue plasminogen activator injection, and photocoagulation. Am J Ophthalmol. 2002 Oct;134(4):609–11. 41. Weiss JN:Treatment of central retinal vein occlusion by injection of tissue plasminogen activator into a retinal vein.Am J Ophthalmol.1998;126(1):142–4. 10.1016/S0002-9394(98)00086-5 42. Kang JW, Yoo R, Jo YH, et al. :Correlation Of Microvascular Structures On Optical Coherence Tomography Angiography With Visual Acuity In Retinal Vein Occlusion.Retina.2017;37(9):1700–9. 43.Nicholson L, Vazquez-Alfageme C, Patrao NV, et al. :Retinal Nonperfusion in the Posterior Pole Is Associated With Increased Risk of Neovascularization in Central Retinal Vein Occlusion.Am J Ophthalmol.2017;182:118–25. 10.1016/j.ajo.2017.07.015 44. Singer MA, Jansen ME, Tyler L, et al. :Long-term results of combination therapy using anti-VEGF agents and dexamethasone intravitreal implant for retinal vein occlusion: an investigational case series.Clin Ophthalmol.2016;11:31–38. 45. Campochiaro PA, Wykoff CC, Brown DM, et al. :Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study.Ophthalmol Retina.2018;2(4):320–328.
2.jpeg)

