Introduction
Papilledema is used to describe optic disc edema resulting from increased intracranial pressure while disc edema could be due to any cause. While disc edema can be unilateral or bilateral, papilledema is usually bilateral, however, it can be asymmetric, to begin with. Another important differential of true papilledema is pseudo papilledema which is seen in conditions like a small hyperopic optic disc or optic disc drusen, etc. This chapter shall entail a discussion on the etiopathogenesis of papilledema and the approach to diagnosis and management of papilledema.
Papilledema and its mechanism
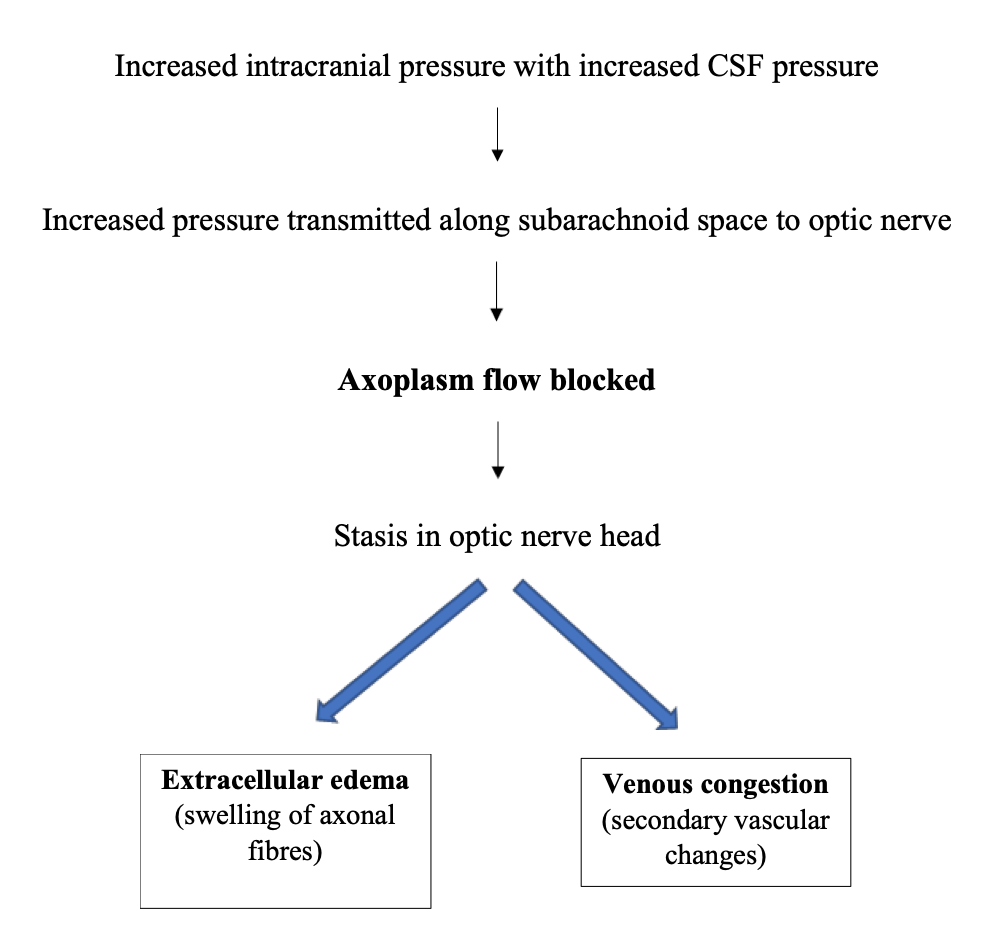
It’s a form of bilateral optic disc swelling associated with increased intracranial pressure. An increase in intracranial pressure can occur secondary to an intracranial space-occupying lesion, cerebral venous thrombosis, or can be idiopathic as in the case of Idiopathic Intracranial hypertension (IIH). Figure 1 depicts the mechanism of the development of papilledema.
Pseudo vs true papilledema
Pseudo papilledema is quite commonly encountered with optic disc drusen being the most frequently observed association. Other causes could be “crowded” hyperopic discs and tilted or anomalous discs, hyaloid traction on the optic disc, and epipapillary glial tissue at the optic disc. Ophthalmoscopic features of pseudo disc edema are enlisted in Table 1.1
Table 1: Features of pseudo disc edema
1. Small disc diameter with small cupping or no cupping.
2. Vessels arising from the center of the disc with well-defined disc margin vasculature.
3. Anomalous branching of vessels (e.g., bifurcations, trifurcations).
4. Visibly exposed drusen if present with or without associated subretinal hemorrhage.
5. Irregular but distinct optic disc margins
6. No superficial capillary telangiectasia on the optic disc head.
7. No hemorrhages or exudates and cotton-wool spots.
In doubtful cases, investigations like ultrasound B scan can be used to confirm the presence of optic disc drusen. They are seen as a hyperreflective echo on a B scan. Apart from that, a CT scan may show hyperintense foci due to calcifications and drusen may autofluorescence on pre-injection-controlled fundus photos. In a retrospective study, ultrasonography was found to be 90% sensitive in detecting papilledema but less specific (79%) in differentiating pseudo papilledema from papilledema in a subset of adult patients who were under evaluation of suspected papilledema over 14 years follow up.2 Ultrasound B scan was considered positive when the optic nerve sheath diameter was ≥ 3.3 mm along with a positive 30-degree test which implied a 10 percent reduction in diameter on A-scan measurement in the presence of excess fluid but no change in the normal optic nerve.
Optical coherence tomography (OCT) is another new tool, that can be used to differentiate between the two conditions.3 Spectral-domain OCT (SD-OCT) is a good diagnostic modality that can detect buried disc drusen.4
Clinical features of papilledema
Clinical symptoms and signs are usually due to underlying CNS pathology that has resulted in increased intracranial pressure
1. Headache- early morning headache waking up the patient from sleep is a characteristic feature. It usually intensifies with head movement, bending, or coughing. The patient can have nausea and projectile vomiting along with it.
2. Visual symptoms- transient vision loss lasting seconds may occur due to the edema causing transient optic nerve ischemia, thereby obscuring the vision. Visual loss usually occurs in the late stages. Horizontal diplopia can occur due to stretching of one or both abducens nerve at the petrous tip and is a false localizing sign. Visual field defects such as enlarged blind spots, generalized constriction, glaucomatous-like defects, and nasal peripheral constriction can also be seen. Afferent pupillary defects are usually not seen unless the patient has severe, asymmetric disc edema
3. Neurological symptoms- the patient can also have other neurological features of underlying CNS pathology like seizures, loss of consciousness, etc.
Stages and frisen5,6,7 grading of papilledema are given in table 2 and table 3 respectively.
Table 2: Grades of papilledema
Early papilledema.
- Minimal disc hyperemia, mild disc elevation with preserved optic cup
- Loss of peripapillary superficial linear and curvilinear light reflex.
- Absence of venous pulsations.
Established papilledema
- Severe disc hyperemia with moderate disc elevation and indistinct margins with the absence of physiological cup
- Engorged and tortuous retinal veins with obscuration of surface vessels by opaque nerve fiber
- splinter hemorrhages at or adjacent to the disc margin
- Paton’s lines (circumferential retinal folds) or choroidal folds.
- Cotton wool spots with or without exudates at the macula (e.g., macular star).
Chronic papilledema
- slow resolution of Haemorrhages and exudates
- milky Gray appearance of the disc.
- drusen-like crystalline deposits (corpora amylacea) on the disc surface.
- Visual field loss including nerve fiber layer defects.
- Optociliary “shunt” (collaterals) vessels on optic nerve head.
• Atrophic papilledema
- Optic disc pallor with nerve fiber bundles visual field defects.
- Attenuation and sheathing of retinal vessels.
- Occasional pigmentary changes or choroidal folds in the macula.
- Sparing of central axons with selective loss of peripheral axons (usually preservation of good central visual acuity).
Table 3: Frisen grading of papilledema
Grade 1:
- C-shaped peripapillary halo with the temporal gap.
- No elevation of the disc margins. Disruption of adjacent radial nerve fiber layer arrangement with greyish opacity accentuating nerve fiber bundles.
Grade 2:
- Complete peripapillary halo.
- Elevation of the nasal border.
Grade 3:
- Elevation of all borders.
- Increased optic nerve head diameter.
- Obscuration of one or more segments of major blood vessels leaving the disc.
Grade 4:
- Obscuration of all borders of disc.
- Elevation of optic nerve head and cup.
- Complete obscuration of a segment of a major blood vessel on the disc.
Grade 5:
- Partial obscuration of all blood vessels on the optic nerve head with total obscuration of at least one major blood vessel.
Causes of secondary intracranial hypertension
Secondary IIH can occur due to cerebral venous obstructive diseases, use of medications like doxycycline,8 nalidixic acid,9 ofloxacin,10 ciprofloxacin11, various endocrine disorders, and hypercoagulable states. Table 4 enlist causes of secondary IIH.
Table 4: causes of secondary IIH
Venous Abnormalities
- Cerebral venous sinus thrombosis
- Superior vena cava syndrome
- Cerebral arteriovenous malformation (AVM) and AV fistulas
- Decreased CSF absorption (e.g., previous intracranial infection or hemorrhage)
Medical Conditions
- endocrine disorders– Addison disease. Hypoparathyroidism and pseudohypoparathyroidism. – Hypothyroidism. Hyperthyroidism.
- Cushing disease and post pituitary surgery for Cushing disease.
- Polycystic ovarian syndrome. Rickets.
- Pregnancy (including ectopic pregnancy) and postpartum
Medications
- Antibiotics-tetracycline, minocycline, doxycycline,8 nalidixic acid,9 sulfa drugs, nitrofurantoin, penicillin, ofloxacin,10 ciprofloxacin.11
- Vitamin A and retinoids
- Withdrawal from chronic corticosteroids
Hypercoagulable States
- Protein S deficiency, Activated protein C resistance, Antithrombin III deficiency, and others
Idiopathic intracranial hypertension
IIH needs a special mention as it is one important cause of papilledema. The disease typically affects obese women of childbearing age12, 13 however, the incidence in males is around 10-15%.14 In the rare case it may occur in children where there is usually no gender prediliction15,16,17 Even though men with IIH are less likely to be obese than women, they tend to be more obese than controls14 hence the occurrence of IIH in young thin man should prompt towards a secondary cause of IIH. Mechanism of IIH in centrally obese patients can be explained by the fact that there is a rise in intra-abdominal pressure, which leads to an increase in pleural pressure and cardiac filling pressure, including central venous pressure, subsequently causing an increase in intracranial venous pressure and intracranial pressure.18 Modified Dandy criteria which involve normal neuroimaging, elevated CSF opening pressures19 with normal CSF contents along with signs and symptoms are used for diagnosis of IIH. Table 5 shows the Modified Dandy criteria for IIH diagnosis
Table 5: Modified Dandy criteria for IIH diagnosis
(1) normal neuroimaging studies (usually MRI/MRV) with no evidence of hydrocephalus, mass lesion, meningeal enhancement, or venous occlusive disease. MRI can show slit-like ventricles and empty sella.
(2) normal CSF contents (including protein and glucose) with no cytologic abnormalities;
(3) elevated opening pressure (values greater than 250 mm H2 O)19
(4) signs and symptoms related only to increased intracranial pressure like headache, papilledema, nonlocalizing sixth nerve palsy.
Investigations in patients with papilledema
All patients with papilledema require a thorough neuroophthalmological history and physical examination. Complete ophthalmologic examination including visual acuity assessment and perimetry (Goldman and/or automated) should be done. OCT of the optic nerve can be done to quantitate papilledema and it can be used along with frisen scale to monitor papilledema over time.
Systemic evaluation of the patient should be done to rule out malignant hypertension as it can cause bilateral disc edema.20 In cases with associated retinal findings like intraretinal hemorrhage or subretinal hemorrhage, venous occlusion; investigations to identify blood dyscrasias should be performed.
Neuroimaging must be done in all patients of papilledema, the choice of which can be based on the history and systemic examination. In acute vascular events like subarachnoid, epidural, subdural, or intracerebral hemorrhage, acute infarction) or in acute head trauma (e.g., assess for fracture, acute bleed) CT imaging is the preferred study. In the rest of the cases, MRI is the investigation of choice in papilledema. MR venography is recommended concurrent to the MRI if there are no lesions on the MRI to evaluate for venous sinus obstruction. If neuroimaging shows no structural lesion or hydrocephalus and IIH is suspected, then lumbar puncture is warranted. Accurate opening pressure, cerebrospinal fluid (CSF) cell count and differential, glucose, protein, cytology, venereal disease research laboratory test, and appropriate studies for microbial agents should be included in the lumbar puncture testing.
Management of papilledema
There are two major goals of treatment of increased intracranial hypertension: the alleviation of symptoms and preservation of visual function. Any underlying cause should be identified and treated and where possible, causative agents should be eliminated. Conditions that predispose to CVS thrombosis and associated complications need to be addressed and anticoagulant therapy should be considered if evaluation confirms a CVS thrombosis. However, in cases of idiopathic intracranial hypertension medical and surgical treatment is considered.
Medical management of IIH
A combination of, weight loss, pharmacotherapy, and diet constitutes medical therapy, with weight loss being the most effective long-term therapy.
A. Weight loss
Weight reduction, by either diet control or surgically in morbidly obese patients, may reduce intracranial pressure and associated papilledema.21 Even a modest amount of weight loss (5–15% of total body weight) can often improve signs and symptoms of IIH. 22 In one study it was found that bariatric surgery when used as a procedure of choice for severely obese patients with IIH, revealed a much higher rate of success than CSF-peritoneal shunting and also provided resolution of additional obesity comorbidity.23
Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), was the first randomized, double-masked, placebo-controlled trial of medical treatment for IIH, in which one group received oral acetazolamide and the other group placebo, with weight loss therapy given in both the groups. It was found that acetazolamide therapy combined with weight loss was safe and effective in IIH patients with mild visual loss at presentation. Also, the placebo group showed significant improvement intensifying the role of weight loss in IIH management.24
B. Drug therapy
Acetazolamide is the first-line drug of choice in patients with mild to moderate visual loss in addition to a low-sodium weight reduction diet. Loop diuretics and topiramate are two other medication options. The initial dose of acetazolamideis 500 mg twice daily and the dose is gradually escalated to 2-4 gm per day depending upon the persistence of papilledema and symptoms and drug tolerance.25 Acetazolamide use should be avoided if possible, during pregnancy, especially before 20 weeks, due to potential teratogenic effects demonstrated in animal studies. The teratogenic effect in humans is not well established, so risk versus benefit must be considered in the individual patient. Caloric restriction and diuretic use, in general, are relatively contraindicated during pregnancy.
Furosemide as a second-line alternative is used in patients who cannot tolerate, are noncompliant with, or fail acetazolamide therapy. Furosemide inhibits CSF production and may have an additive effect with acetazolamide. It is administered initially as 20 mg twice daily, to a maximum of 40 mg three times daily when needed.
Topiramate is also used as a drug of choice for managing headaches. This along with its weight loss-causing ability makes it a useful option for IIH treatment. Use of corticosteroids is usually avoided even though some initial benefit is seen, due to associated complications, including weight gain in the chronic treatment of an obese patient population and the associated risk of rebound IIH.26
Surgical management of IIH
When the patient is not responding to maximum medical therapy and there is a threat of severe or permanent visual dysfunction without prompt intervention, surgical treatment is indicated.27 The options for surgical management include CSF diversion (lumboperitoneal shunt [LPS] or ventricular peritoneal shunt [VPS]), Optic Nerve Sheath Fenestration (ONSF), and venous sinus stenting. Table 6 enlist indications for surgery in IIH patients.
Table 6: Indications for surgery
- New visual field defect.
- Enlargement of previously existing visual field defect.
- Reduced visual acuity without any macular edema.
- Presence of severe visual loss (20/40 or worse) in one or both eyes at the time of initial examination.
- Psychosocial reasons, such as patient’s inability to perform visual field studies, noncompliance with medications, or itinerant lifestyle.
A. CSF diversion procedures
They include lumboperitoneal and ventriculoperitoneal shunts. CSF diversion can relieve headache, diplopia, and papilledema, and can reverse visual loss.28,29 These procedures can also be performed in pregnancy.30 Performing an LPS would obviate the need for an intracranial procedure (ventricular shunt) and shall spare the patient from associated risks (e.g., intracranial hemorrhage), but an LPS is more likely to require revision or conversion to a ventricular shunt.31,32 However, with the advent of stereotactic neuronavigation ventricular shunts are being increasingly performed.33 LPS failure is common, and most shunt failures occur within 2 to 3 months of the initial LPS (cumulative risk, 37%)34
Thus, CSF diversion procedures are often effective in controlling the raised intracranial pressure, and although the placement of the shunt is generally safe, any operation performed under general anesthesia carries inherent risk, and there is at least one perioperative death reported following LPS.35 Shunt obstruction is the most common complication followed by secondary intracranial hypotension caused by excessive drainage of the CSF via the LPS.34 Both VPS and LPS carry a risk of post-procedure headaches that need to be considered when approaching a patient with already intractable headaches.
B. Optic nerve sheath fenestration
Optic nerve sheath fenestration can be used in patients non-responsive to medical treatment and can help to prevent deterioration in vision and, in some cases, improve visual function in patients with IIH. 36,37 ONSF was found to improve visual acuity, headache, and papilledema in 36.5, 70, and 90% of reported cases, respectively in a study conducted by Lai et al.38 However possible post-operative complications include ocular motility disorders, optic nerve trauma, orbital hemorrhage, orbital apex syndrome, etc.
C. Venous stenting
This procedure can be undertaken in patients with stenosed dural sinus causing secondary IIH. However, with venous stenting as well, life-threatening complications like intracranial hemorrhage can occur creating a need for urgent neurosurgery39
Summary
- Papilledema is bilateral optic disc swelling due to increased intracranial pressure.
- It is important to distinguish between pseudo disc edema and true disc edema as the latter may require urgent management.
- All papilledema patients should undergo neuroimaging so that the associated CNS pathology is not missed.
- In typical cases of IIH, weight loss therapy along with acetazolamide should be undertaken as first-line therapy.
- In atypical cases of IIH, thorough history, ocular examination, and systemic workup should be done to rule out a secondary cause of IIH.

Figure 2 demonstrates an approach to the management of bilateral disc edema.
References:
- Glaser JS. Neuro-ophthalmology. 2nd ed. Philadelphia, PA: JP Lippincott; 1990:106)
- Carter SB, Pistilli M, Livingston KG, et al. The role of orbital ultrasonography in distinguishing papilledema from pseudopapilledema. Eye (Lond). 2014; 28(12):1425–1430).
- Auinger P, Durbin M, Feldon S, et al. OCT Sub-Study Committee for NORDIC Idiopathic Intracranial Hypertension Study Group. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci. 2014; 55(12):8173–8179
- Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009; 127(1):45–49)
- Friedman DI. Papilledema and pseudotumor cerebri. Ophthalmol Clin North Am. 2001; 14(1):129–147, ix
- Scott CJ, Kardon RH, Lee AG, Frisén L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010; 128(6):705–711
- Fischer WS, Wall M, McDermott MP, Kupersmith MJ, Feldon SE, NORDIC Idiopathic Intracranial Hypertension Study Group. Photographic reading center of the idiopathic intracranial hypertension treatment trial (IIHTT): methods and baseline results. Invest Ophthalmol Vis Sci. 2015; 56(5): 3292–3303)
- Friedman DI. Medication-induced intracranial hypertension in dermatology. Am J Clin Dermatol. 2005; 6(1):29–37
- Mukherjee A, Dutta P, Lahiri M, Sinha S, Mitra AK, Bhattacharya SK. Benign intracranial hypertension after nalidixic acid overdose in infants. Lancet. 1990; 335(8705):1602
- Getenet JC, Croisile B, Vighetto A, et al. Idiopathic intracranial hypertension after ofloxacin treatment. Acta Neurol Scand. 1993; 87(6):503–504
- Winrow AP, Supramaniam G. Benign intracranial hypertension after ciprofloxacin administration. Arch Dis Child. 1990; 65(10):1165–1166
- Lai LT, Danesh-Meyer HV, Kaye AH. Visual outcomes and headache following interventions for idiopathic intracranial hypertension. J Clin Neurosci. 2014; 21(10):1670–1678
- Spitze A, Malik A, Al-Zubidi N, Golnik K, Lee AG. Optic nerve sheath fenestration vs cerebrospinal diversion procedures: what is the preferred surgical procedure for the treatment of idiopathic intracranial hypertension failing maximum medical therapy? J Neuroophthalmol. 2013; 33(2):183– 188)}
- Digre KB, Corbett JJ. Pseudotumor cerebri in men. Arch Neurol. 1988; 45(8): 866–872)
- Balcer LJ, Liu GT, Forman S, et al. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999; 52(4):870–872
- Cinciripini GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J Ophthalmol. 1999; 127(2):178–182
- Lessell S. Pediatric pseudotumor cerebri (idiopathic intracranial hypertension). Surv Ophthalmol. 1992; 37(3):155–166)
- Sugerman HJ, DeMaria EJ, Felton WL, III, Nakatsuka M, Sismanis A. Increased intra-abdominal pressure and cardiac filling pressures in obesity associated pseudotumor cerebri. Neurology. 1997; 49(2):507–511)
- Corbett JJ, Mehta MP. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983; 33(10):1386–1388)
- Lee AG, Beaver HA. Acute bilateral optic disk edema with a macular star figure in a 12-year-old girl. Surv Ophthalmol. 2002; 47(1):42–49)
- Fridley J, Foroozan R, Sherman V, Brandt ML, Yoshor D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg. 2011; 114(1):34–39
- Wong R, Madill SA, Pandey P, Riordan-Eva P. Idiopathic intracranial hypertension: the association between weight loss and the requirement for systemic treatment. BMC Ophthalmol. 2007;
- Sugerman HJ, Felton WL, III, Sismanis A, Kellum JM, DeMaria EJ, Sugerman EL. Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann Surg. 1999; 229(5):634–640, discussion 640–642
- Wall M, McDermott MP, Kieburtz KD, et al. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014; 311(16):1641–1651
- Schoeman JF. Childhood pseudotumor cerebri: clinical and intracranial pressure response to acetazolamide and furosemide treatment in a case series. J Child Neurol. 1994; 9(2):130–134
- Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension. Arch Neurol. 1989; 46(10):1049–1051
- Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension. Arch Neurol. 1989; 46(10):1049–1051
- Rosenberg ML, Corbett JJ, Smith C, et al. Cerebrospinal fluid diversion procedures in pseudotumor cerebri. Neurology. 1993; 43(6):1071–1072
- [315] Rowe FJ. Assessment of visual function in idiopathic intracranial hypertension. Br J Neurosurg. 2011; 25(1):45–54
- Shapiro S, Yee R, Brown H. Surgical management of pseudotumor cerebri in pregnancy: case report. Neurosurgery. 1995; 37(4):829–831
- Abubaker K, Ali Z, Raza K, Bolger C, Rawluk D, O’Brien D. Idiopathic intracranial hypertension: lumboperitoneal shunts versus ventriculoperitoneal shunts–case series and literature review. Br J Neurosurg. 2011; 25(1):94–99
- [320] McGirt MJ, Woodworth G, Thomas G, Miller N, Williams M, Rigamonti D. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg. 2004; 101(4):627–632
- Tulipan N, Lavin PJ, Copeland M. Stereotactic ventriculoperitoneal shunt for idiopathic intracranial hypertension: technical note. Neurosurgery. 1998; 43 (1):175–176, discussion 176–177
- Eggenberger ER, Miller NR, Vitale S. Lumboperitoneal shunt for the treatment of pseudotumor cerebri. Neurology. 1996; 46(6):1524–1530
- Eisenberg HM, Davidson RI, Shillito J, Jr. Lumboperitoneal shunts. Review of 34 cases. J Neurosurg. 1971; 35(4):427–431
- Spoor TC, McHenry JG, Shin DH. Long-term results using adjunctive mitomycin C in optic nerve sheath decompression for pseudotumor cerebri. Ophthalmology. 1995; 102(12):2024–2028
- [352] Spoor TC, Ramocki JM, Madion MP, Wilkinson MJ. Treatment of pseudotumor cerebri by primary and secondary optic nerve sheath decompression. Am J Ophthalmol. 1991; 112(2):177–185
- Lai LT, Danesh-Meyer HV, Kaye AH. Visual outcomes and headache following interventions for idiopathic intracranial hypertension. J Clin Neurosci. 2014; 21(10):1670–1678
- Ahmed R, Friedman DI, Halmagyi GM. Stenting of the transverse sinuses in idiopathic intracranial hypertension. J Neuroophthalmol. 2011; 31(4):374–380


