Dacryocystorhinostomy (DCR), a century-old procedure described by Toti in the early 1900s, remains the gold standard for treating Nasolacrimal Duct Obstruction (NLDO).1 For any other procedure related to the treatment of NLDO, they are all compared and evaluated against DCR. The surgical anatomy overlaps the areas of expertise of an ophthalmologist or an oculoplastic specialist and an otolaryngologist, as the majority of the Nasolacrimal duct lies in the nasal cavity. Therefore, both external and endonasal approaches are available. In 1893, Caldwell first described the endonasal approach of DCR,2 and almost a decade later, in 1904, Toti described the external approach of DCR, which became the gold standard for the treatment of NLDO. The main goal of DCR is to create a fistulous tract lined with epithelium from the lacrimal sac into the nasal cavity.
Although the endonasal approach was first described, it did not gain popularity due to the non-availability of nasal endoscopes and difficulty in visualizing anatomical landmarks in the nose, resulting in higher failure rates. However, the development of surgical endoscopes in the early 1970s and 1980s renewed interest in the endonasal approach. Massaro and coworkers 3 described the use of an argon blue-green laser with a trans-canalicular fiber-optic light source to create the osteotomy. Subsequently, many surgeons used various lasers, such as carbon dioxide, potassium titanyl phosphate, holmium: YAG, and neodymium: YAG lasers.4,5,6 However, laser use led to thermal injury to tissues, causing more scarring and thus closure of the osteotomy and failure of the procedure. Most studies from the early 1990s showed a 70-80% success rate with laser endoscopic DCR.7 Javate and associates described the use of radio-frequency to remove bone and mucosa.8 In 2003, Professor Peter Wormald described the technique of using mucosal flaps and a powered diamond burr to remove bone, marking the acceptance of mechanical endonasal DCR.9 Currently, both external and endonasal DCR techniques provide comparable success rates.10,11,12,13 The current chapter primarily focuses on external DCR
Indications for DCR are listed in Table 1. Regardless of the specific indication, the steps of the procedure remain standard, with possible variations between pediatric and adult DCRs. The absolute contraindication for the procedure is the suspicion or intraoperative diagnosis of a lacrimal sac tumor or an adnexal mass extending into the lacrimal sac. Relative contraindications include acute dacryocystitis (which requires a one-month waiting period for the acute episode to subside in external DCR, whereas endoscopic DCR can be performed during acute dacryocystitis), lacrimal abscess, and patient age (less than one year).15
Indications of Dacryocystorhinostomy (DCR)14,15
- Persistent Congenital Nasolacrimal Duct Obstruction (CNLDO) in previous failed therapies
- CNLDO associated with mucocele, dacryocystitis, and not responsive to other treatments
- Epiphora due to Primary Acquired Nasolacrimal Duct Obstruction (PANDO) with Chronic Dacryocystitis
- Mucocele of the lacrimal tear sac
- To relieve infection of the lacrimal sac before intraocular surgery
- Secondary acquired lacrimal duct obstruction (SALDO) due to trauma, infections etc
Preoperative Assessment and History Taking :14,15
Diagnosis of NLDO should be established based on:
- History and clinical symptoms of NLDO, such as onset and duration of watering, discharge
- Presence of ROPLAS (Regurgitation on pressure over lacrimal sac area)
- Syringing (presence of regurgitation from opposite punctum which may have mucoid flakes) and hard-stop on probing
- Careful evaluation of eyelid (rule out entropion, ectropion, lid laxity, inflammation, fistula), punctum (rule to stenosis, agenesis, tumors), canaliculus (rule out stenosis, block, inflammation), and Lacrimal sac area
Syringing should also be performed for the contralateral eye. The above-enlisted diseases may cause persistent epiphora even after a successful DCR and should be considered both preoperatively and postoperatively. Anterior rhinoscopy, with or without nasal endoscopy, should be performed to check for any nasal polyps, masses, discharge, deviated nasal septum, and hypertrophic turbinates. Imaging is usually not necessary except in cases of trauma, naso-orbital fractures, or suspected lacrimal sac tumors. Preoperative computed tomography (CT) scans may be useful for identifying orbital, nasal, or sinus pathologies in such cases. A checklist for ruling out autoimmune disorders such as sarcoidosis and Wegener's granulomatosis should include seeking a history of epistaxis, nasal discharge, respiratory problems, recurrent sinusitis, joint pains, and weight loss
Preliminary tests and requisites14,15
- Confirmation of the diagnosis based on clinical findings and examination.
- Complete blood count with Haemoglobin levels.
- Coagulation profile, including bleeding and clotting time, should be done. Prothrombin time may be done for patients on anti-coagulants. Stop anti-coagulants at least three days before surgery.
- Measurement of blood pressure and strict control of hypertension.
- Measurement of blood sugar.
- Electrocardiogram for the patient over 50 years and smokers.
- Physician clearance for systemic diseases.
- General anesthesia investigations and pre-anesthetist check-up for relevant cases.
- Nasal decongestion using 0.05% Oxymetazoline for adults and 0.025% for children < 4 years. It should be started preferably three days before the planned date of surgery.
Anesthesia
Regional/Local anaesthesia: This is most commonly used for DCR surgery, especially in elderly patients who are not suitable for general anesthesia. It may be combined with sedation. The advantages include early discharge post-surgery, the procedure being manageable as daycare, reduced bleeding, and quicker rehabilitation.
Nerve supply: The superomedial area above the medial canthal tendon region receives innervation from the infratrochlear nerve and the anterior ethmoidal nerve (a terminal branch of the nasociliary nerve). The lower part of the sac and duct on the anterior surface is innervated by the nasociliary nerve and the dorsal nasal nerve. The infraorbital nerves supply the more inferior and lateral aspects. The infratrochlear and nasociliary nerves are branches of the first division of the trigeminal nerve (the Ophthalmic division). The infraorbital nerve is a branch of the second division of the trigeminal nerve (the Maxillary division). The local anesthesia for DCR consists of four parts:
- Ocular anesthesia: Topical conjunctival instillation of proparacaine 0.5% or 2% lignocaine gel
- Nasal anesthesia: Nasal spray of 10% Lignocaine and nasal packing with 4% Lignocaine with 1:2,00,000 Adrenaline soaked gauze
- Nerve blocks: For Infratrochlear, Infraorbital, Anterior ethmoidal, and Dorsal nasal nerve
- Infiltration: Subcutaneous injection along the incision line for hemostasis
Sources of bleeding during DCR:
- Fine vessels in Orbicularis
- Angular vein
- Nutrient artery in the frontal process of the maxilla
- Ethmoid mucosa
- Nasal mucosa
- Bridging Periosteal blood vessels between lacrimal sac and fossa
- Branches of Infraorbital or Infratrochlear artery
Pre-surgery planning: Includes reverse Trendelenburg position (head-up feet-down), Cleaning and draping keeping the eye and nose open, Good illumination, Good suction, and magnification binocular loupe, or some surgeons prefer operating microscope.
SURGICAL STEPS OF DCR
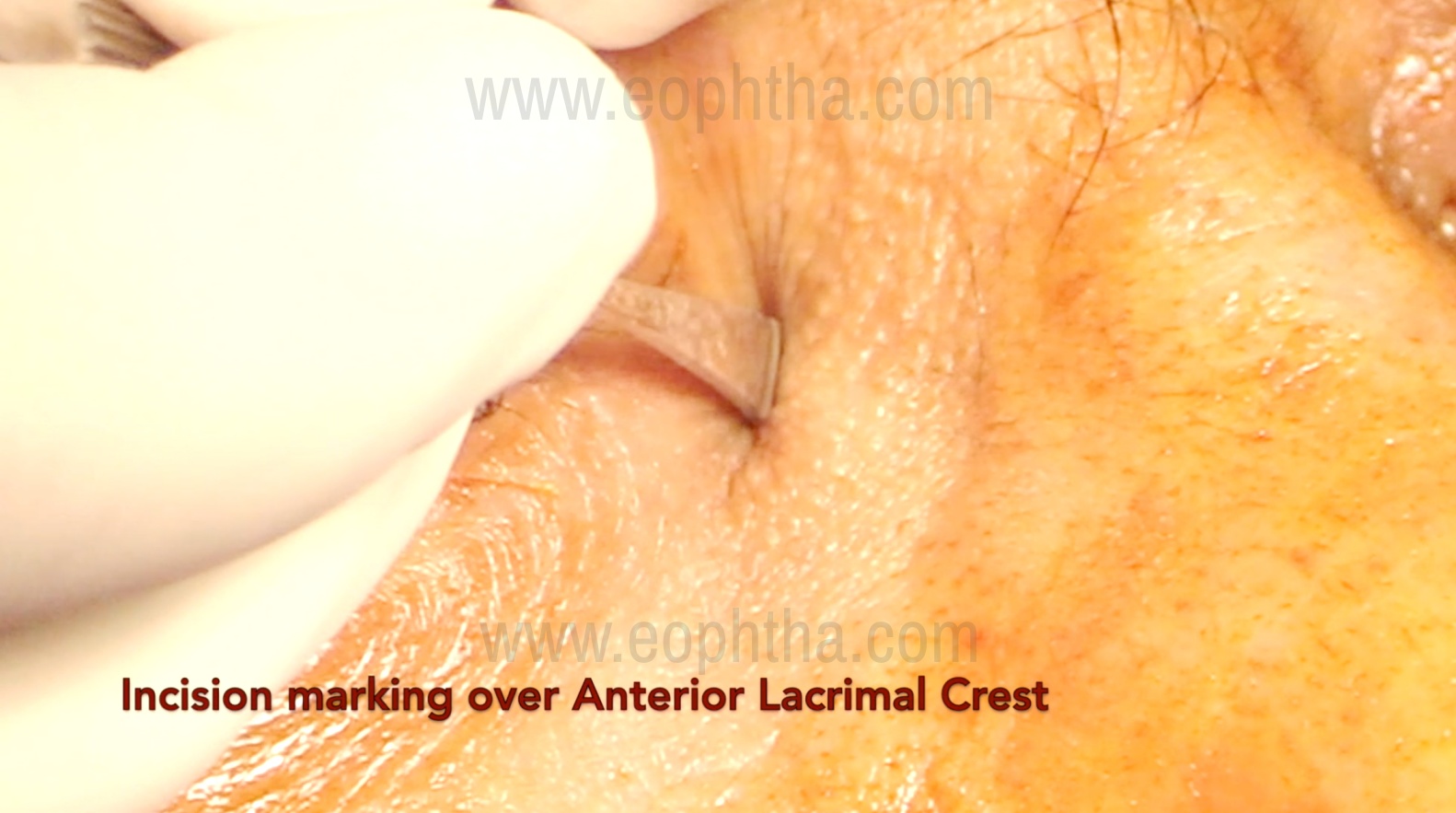
The incision for external DCR (Figure 1 &2)
These are based on surgeon preference: a straight incision made more than 10 mm medial to the medial canthus, medial to the angular vein (the angular vein lies 4-10 mm medial to the medial canthus), and a curvilinear incision 3-4 mm medial to the medial canthus along the anterior lacrimal crest. Skin incisions are usually 10-12 mm in length. The skin incision is made with a no. 11 or 15 Bard-Parker handle
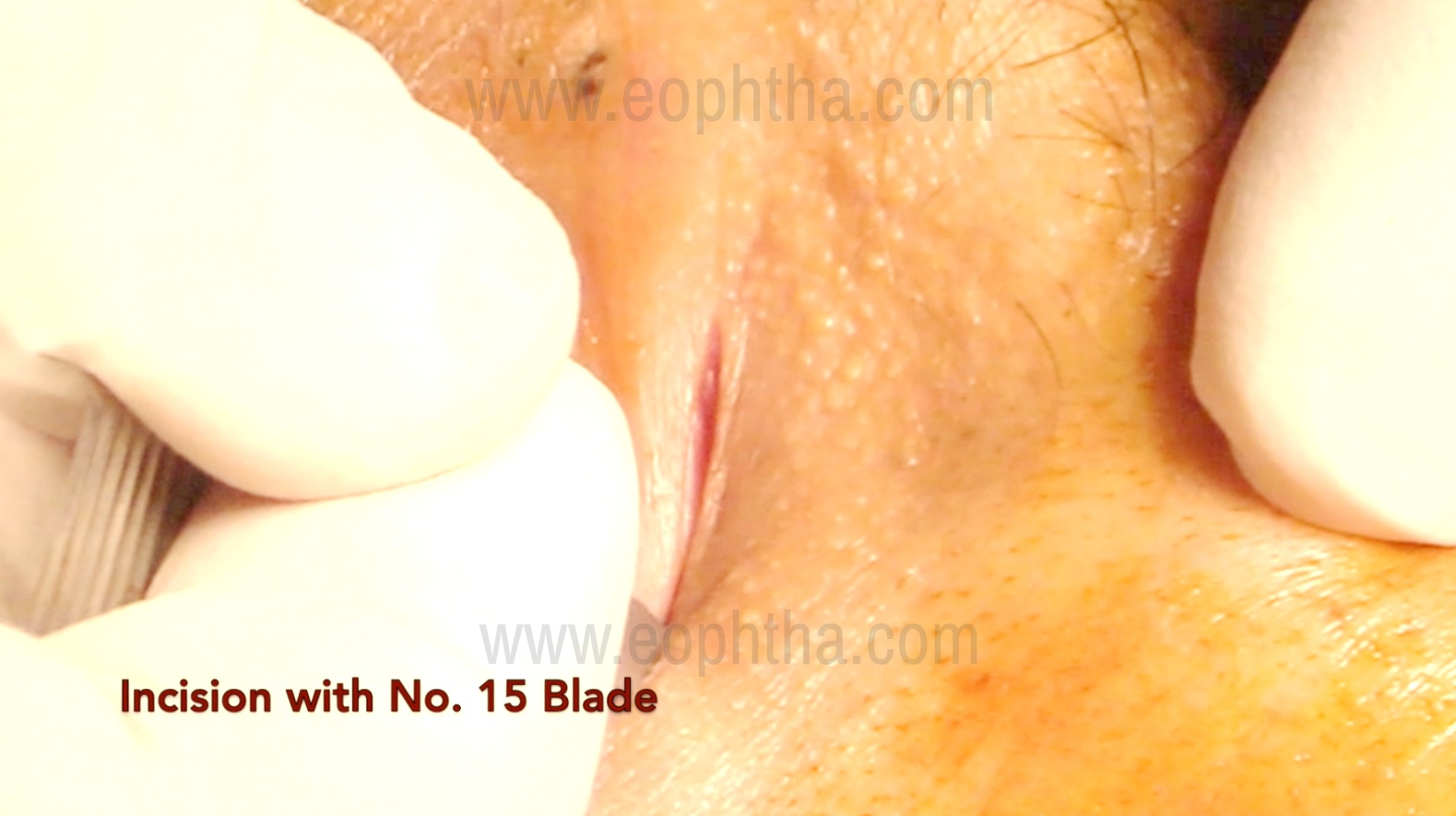
Orbicularis dissection (Figure 3)
After the skin incision, the orbicularis muscle is bluntly dissected using tenotomy scissors, with the blade opening parallel to the muscle fibers to prevent post-operative scarring. Adequate hemostasis is necessary from the start, and it is important to avoid damage to the angular vein during this step. Inadvertent injury to the angular vein can cause hemorrhage, requiring immediate cauterization or suturing of the vessel. A cat's paw retractor is then used to retract the skin and orbicularis muscle on the medial side of the incision. Alternatively, 4-0/5-0 silk sutures may be placed to retract the skin edges, two medially and two laterally.
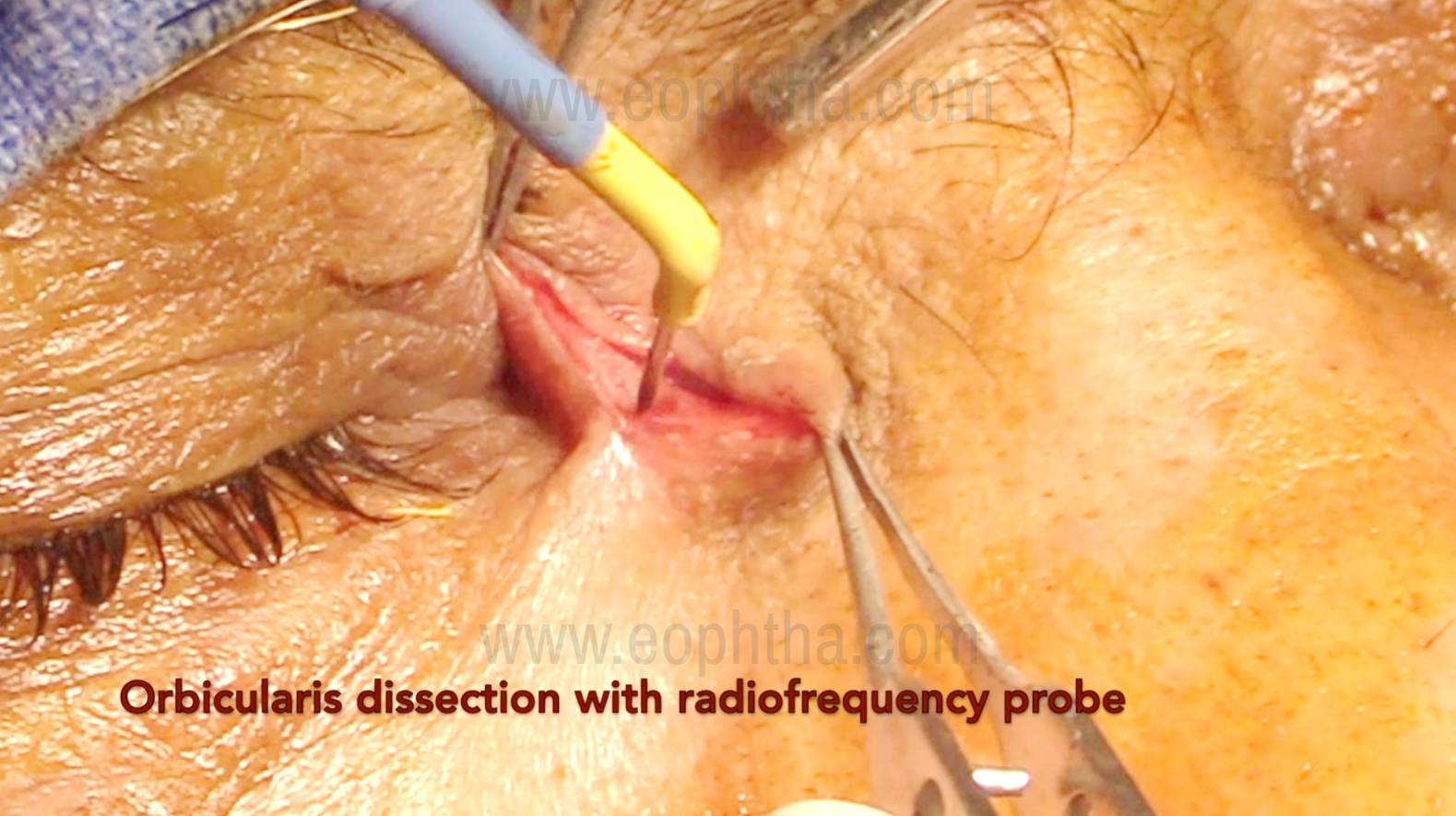
Identification of Medial canthal tendon (Figure 4)
The anterior limb of the medial canthal tendon is easily identified as a horizontal white band running from the medial canthus to the edge of the anterior lacrimal crest medially. It divides the lacrimal sac into the fundus superiorly and the body inferiorly. Cutting the tendon typically aids in the complete exposure of the fundus of the sac and the creation of sufficient superior osteotomy. Cutting the anterior limb of the medial canthal tendon does not lead to eyelid malposition because the posterior limb maintains the eyelid's tightness and posterior direction, apposed to the globe.
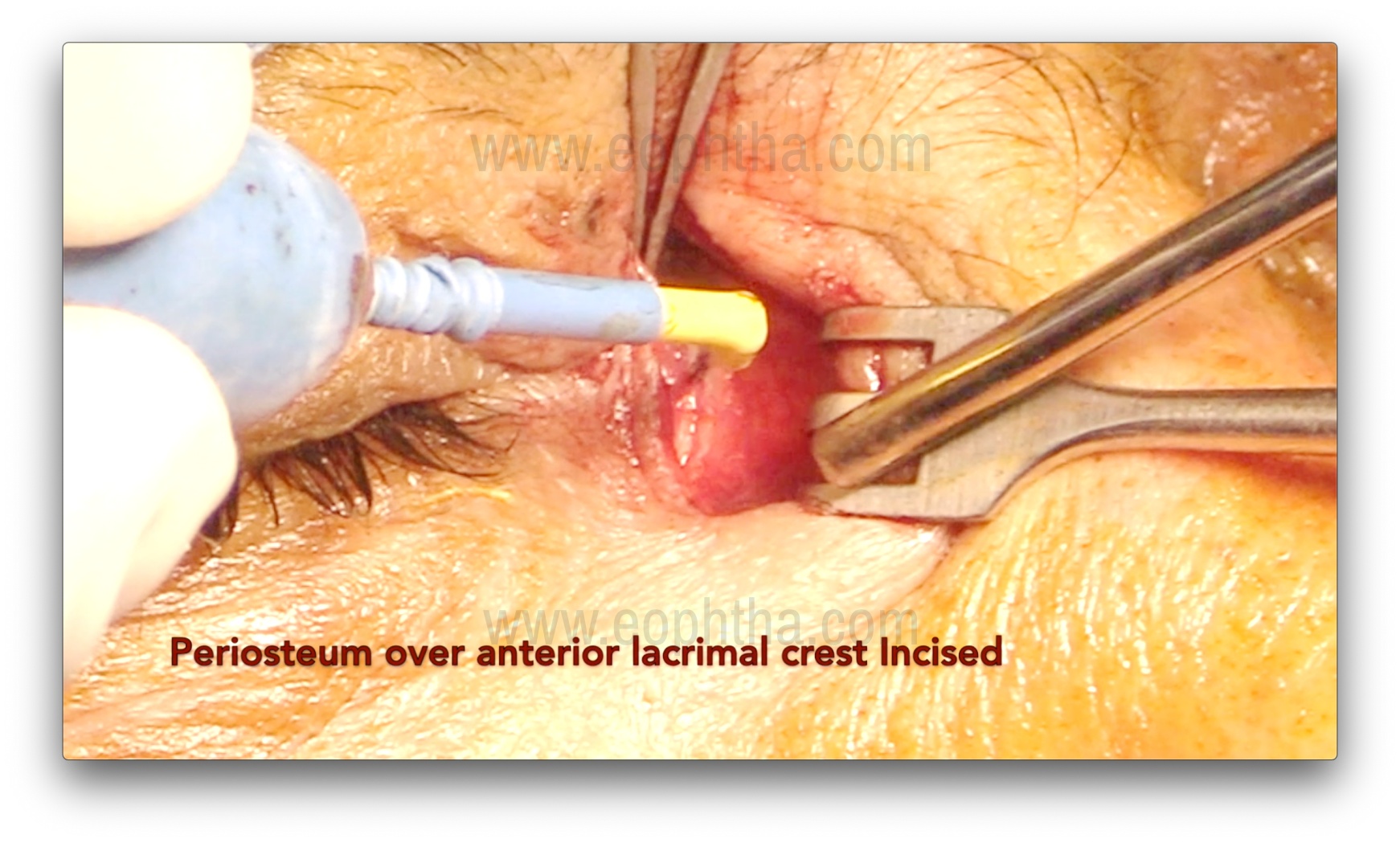
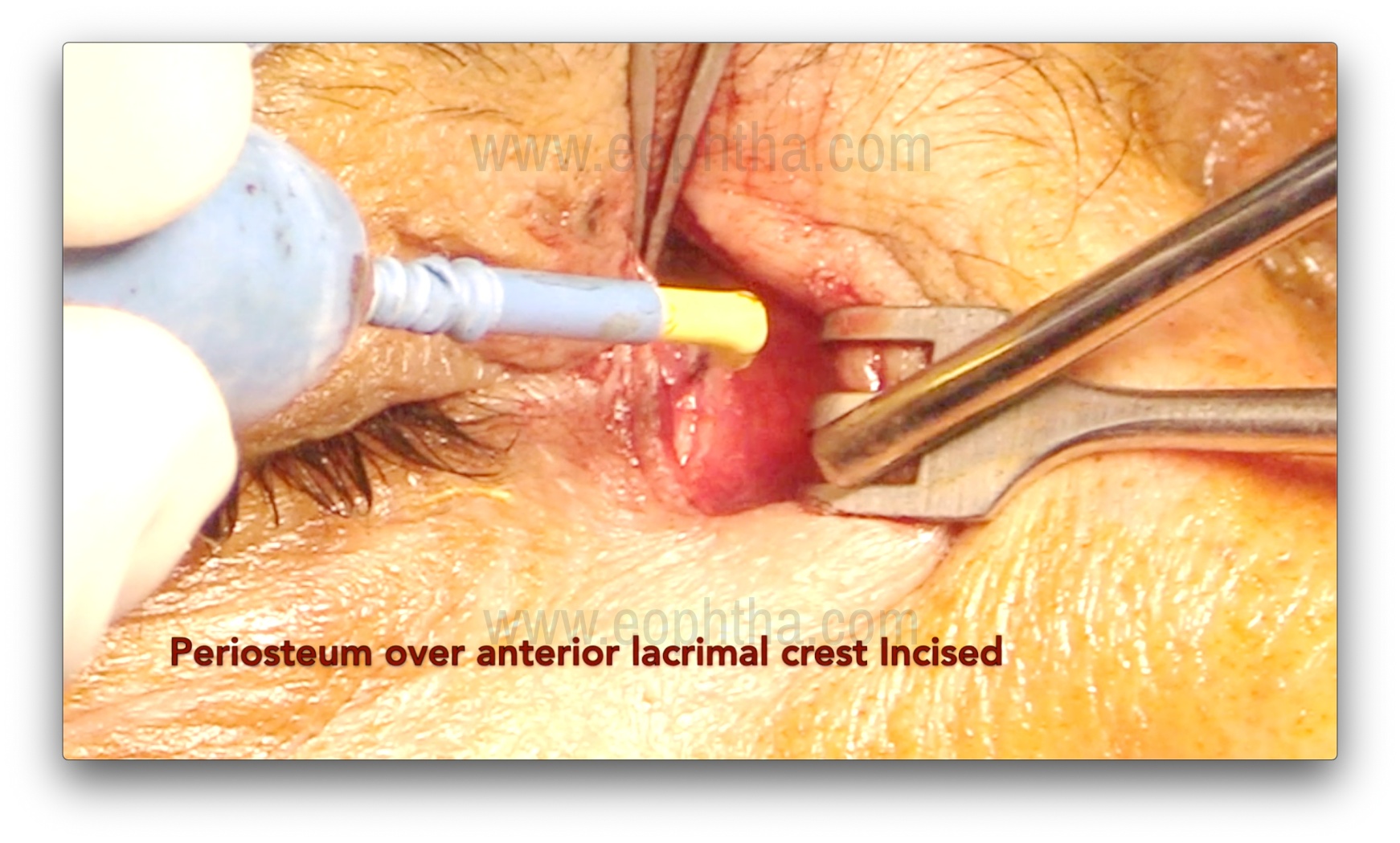
Periosteum incision (Figure 5 & 6)
After careful dissection of the orbicularis, the periosteum over the anterior lacrimal crest is exposed and incised using a B.P. knife or radio-frequency cautery. An incision is made 2 mm anterior to the anterior lacrimal crest. After the incision, the periosteum is lifted using Freer's periosteal elevator over the lacrimal crest. The periosteum is then lifted over the edge of the anterior lacrimal crest, followed by gentle dissection to expose the lacrimal sac and reflect it from the lacrimal sac fossa. Subsequently, the sac is completely separated from the fossa. Bleeding from the nutrient artery, which lies in the frontal process of the maxilla (forming the anterior lacrimal crest), may occur during this step and require cauterization.
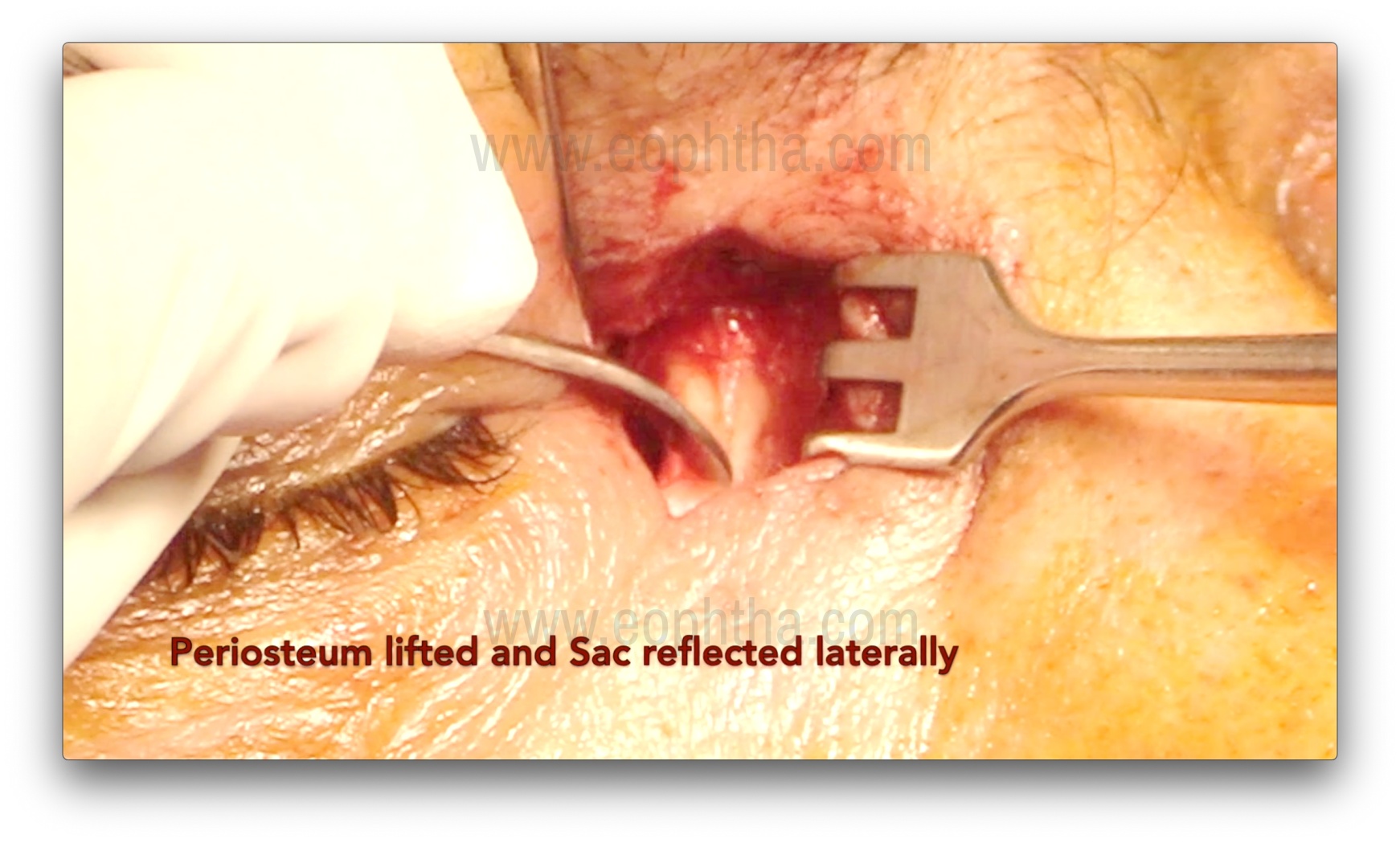
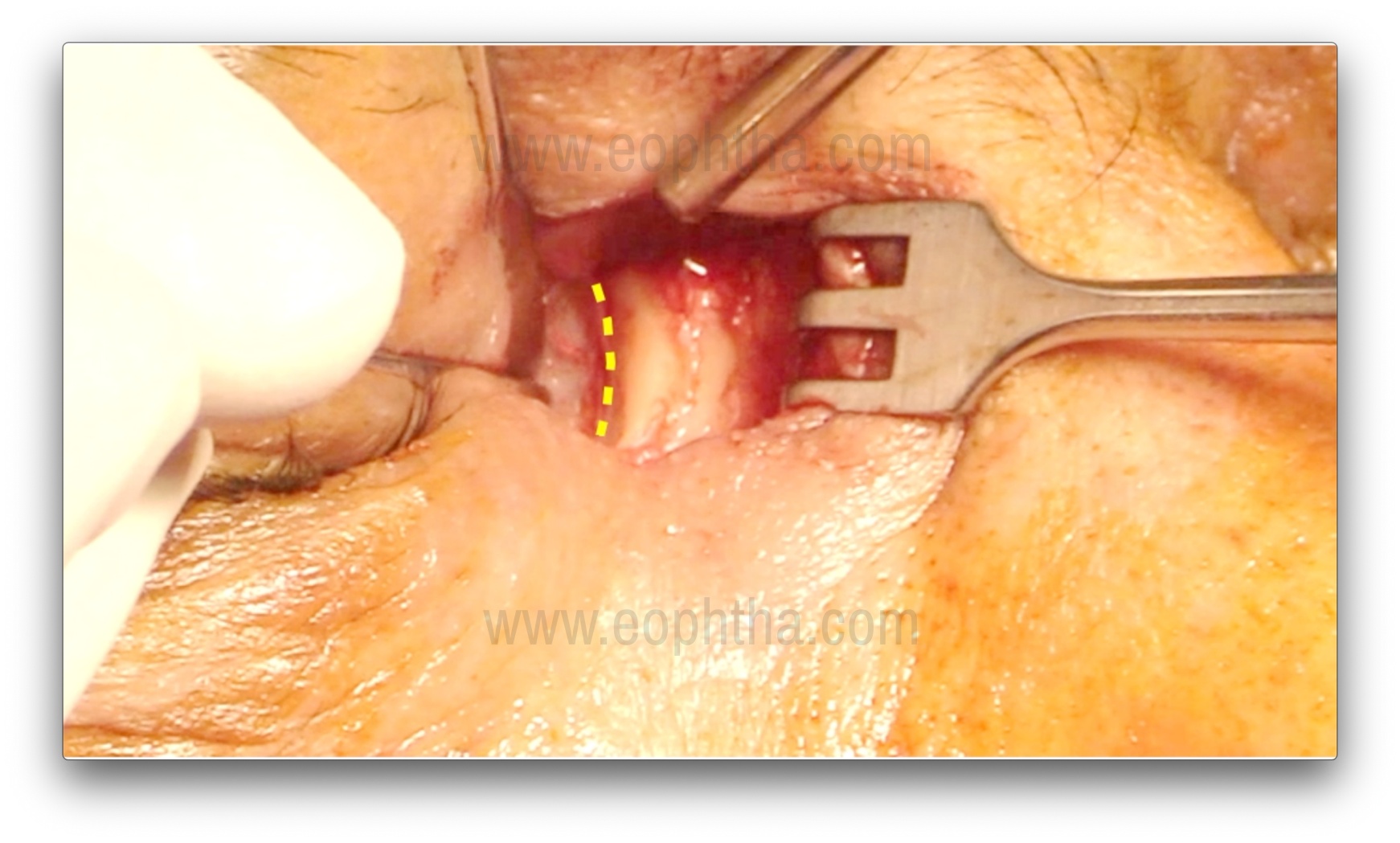
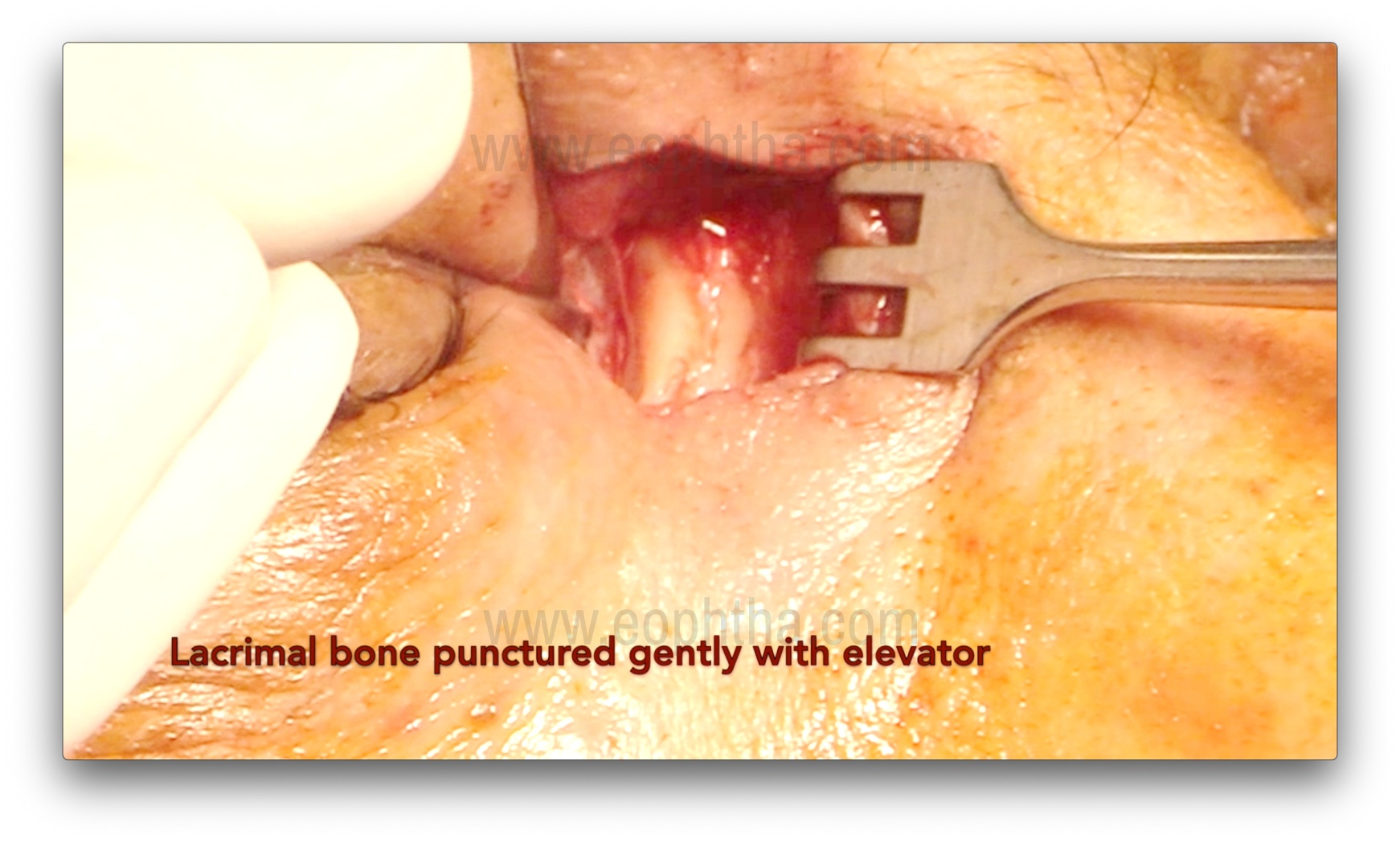
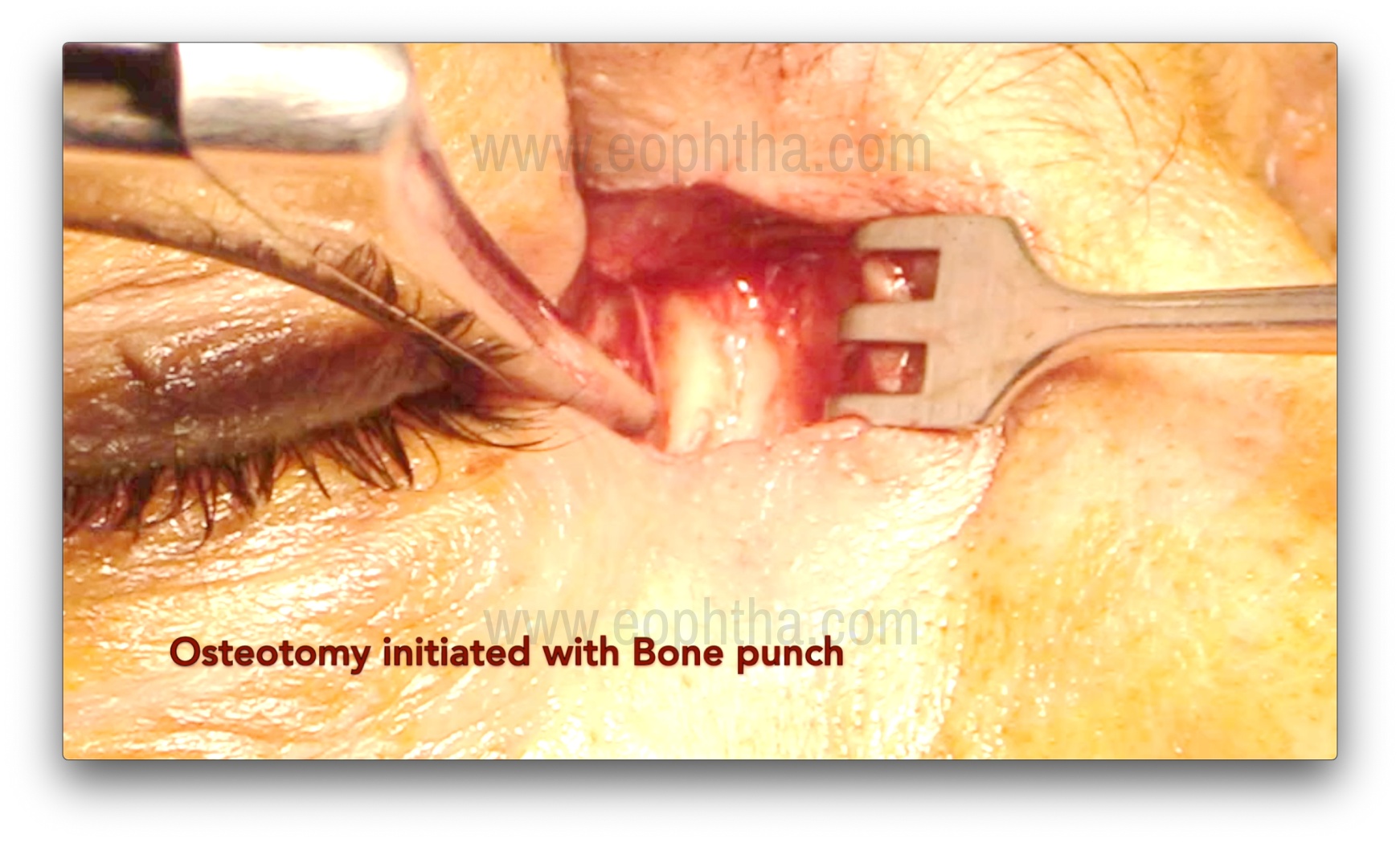
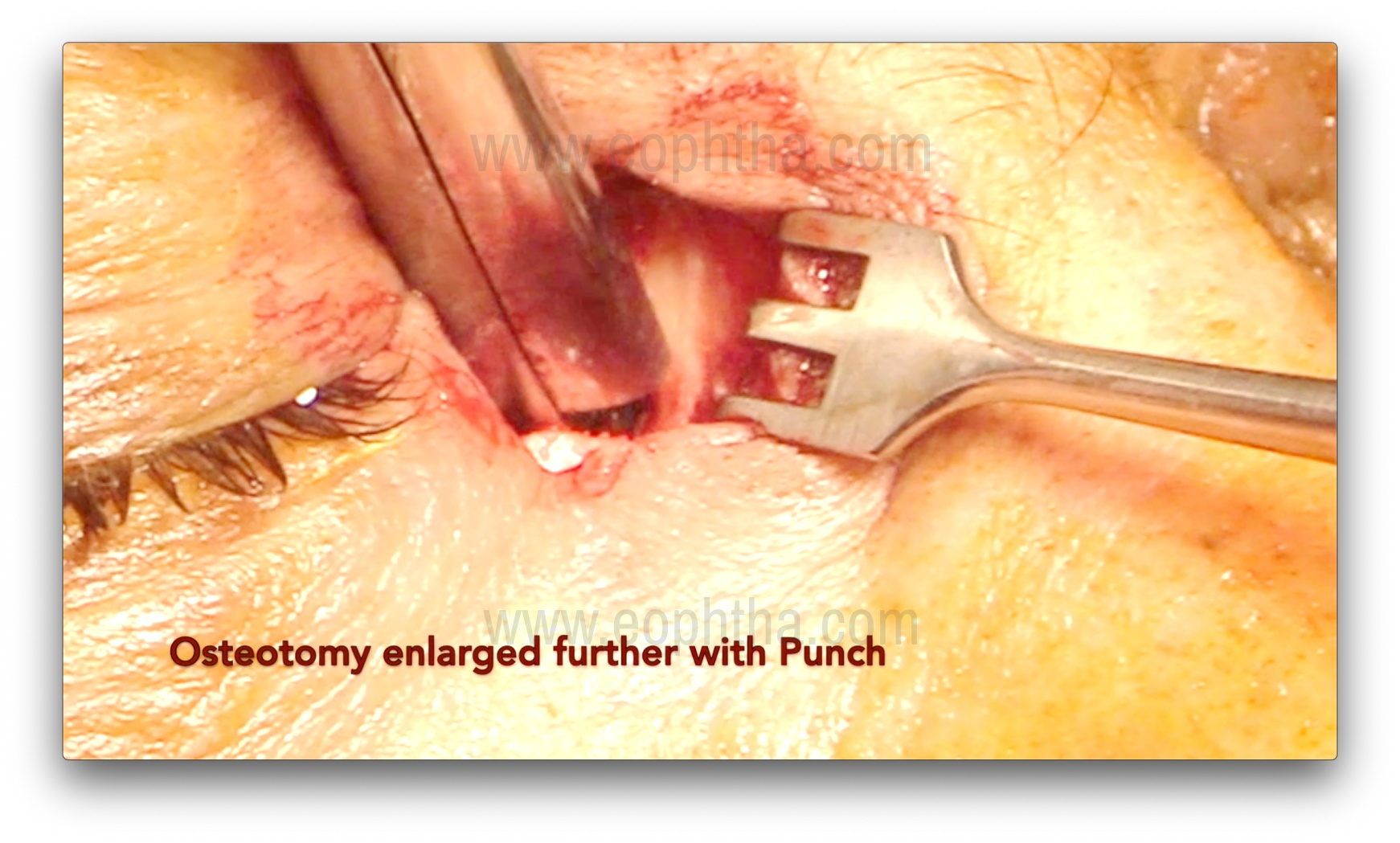
Osteotomy creation (Figure 7, 8, 9 & 10)
After the lacrimal sac is reflected laterally, the junction of the thin lamina papyracea and the ethmoid bone is identified. The thin lamina papyracea appears bluish, while the thicker ethmoid bone appears whitish. The suture line between the lamina papyracea and the ethmoid is then fractured with a periosteal elevator, marking the first step of osteotomy creation. A Kerrison bone rongeur is then used to enlarge the ostium by gently inserting it between the bone and nasal mucosa. This is achieved by gently pushing the mucosa posteriorly and obtaining a cleavage plane between the bone and the mucosa. Care should be taken to avoid entrapment of the mucosa in the rongeur's bite. The sizes of rongeurs used initially are 1-1.5 mm, and with the sequential enlargement of the osteotomy, sizes of 2, 3, and 4 mm are used. The usual size of the osteotomy is about 1.5 cm, but perhaps more important are the anatomical landmarks for the osteotomy.14:
- Anterior removal of frontal process of maxillary bone is till the rongeur cannot be inserted between the bone and nasal mucosa further.
- Posterior extent is till the removal of anterior ethmoid air cells and their thin eggshell bony walls.
- Superior extent is up to 2-5 mm above the medial canthal tendon.
- Inferior extent is till the lower part of lacrimal sac and upper nasolacrimal duct is partly deroofed.
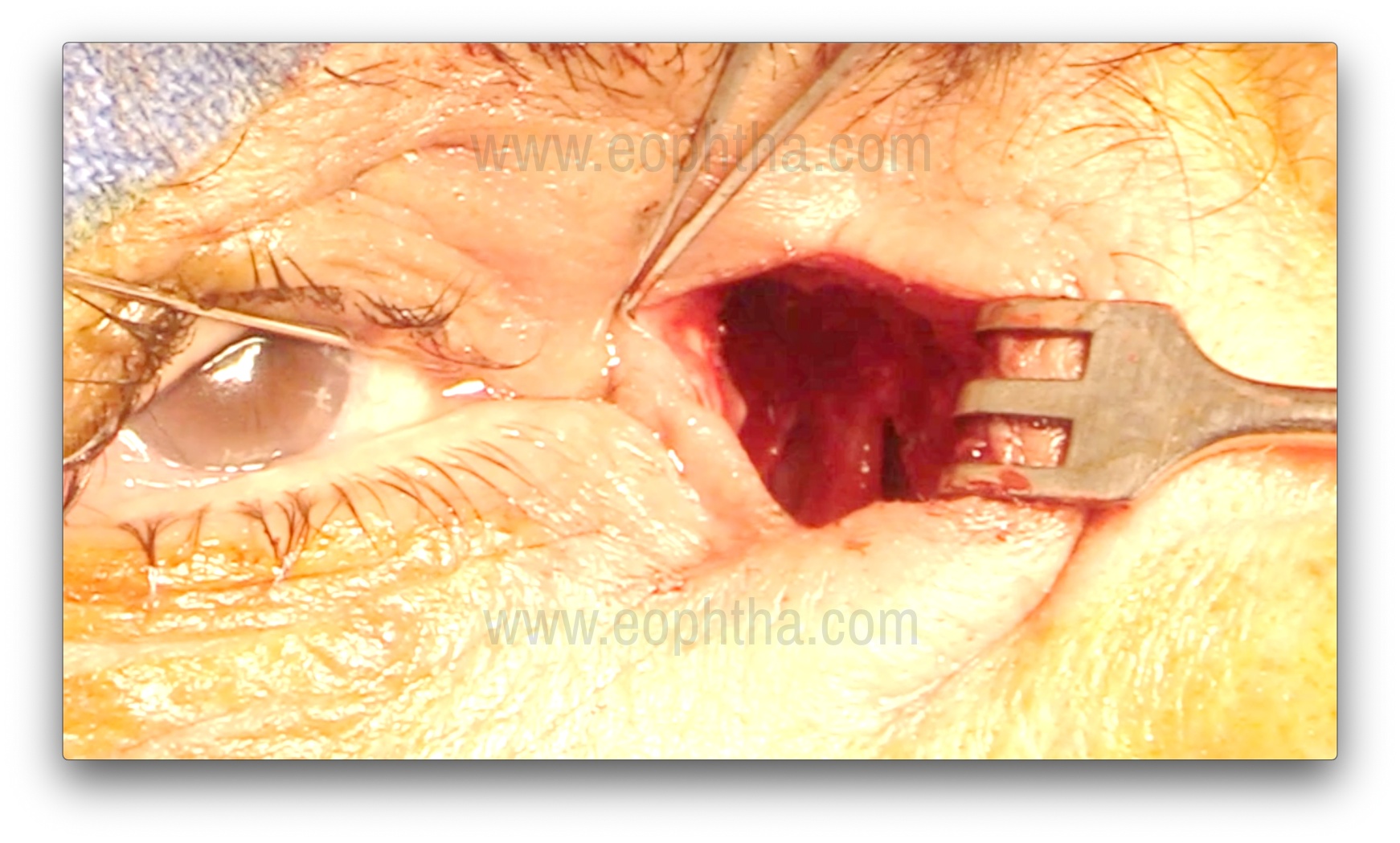
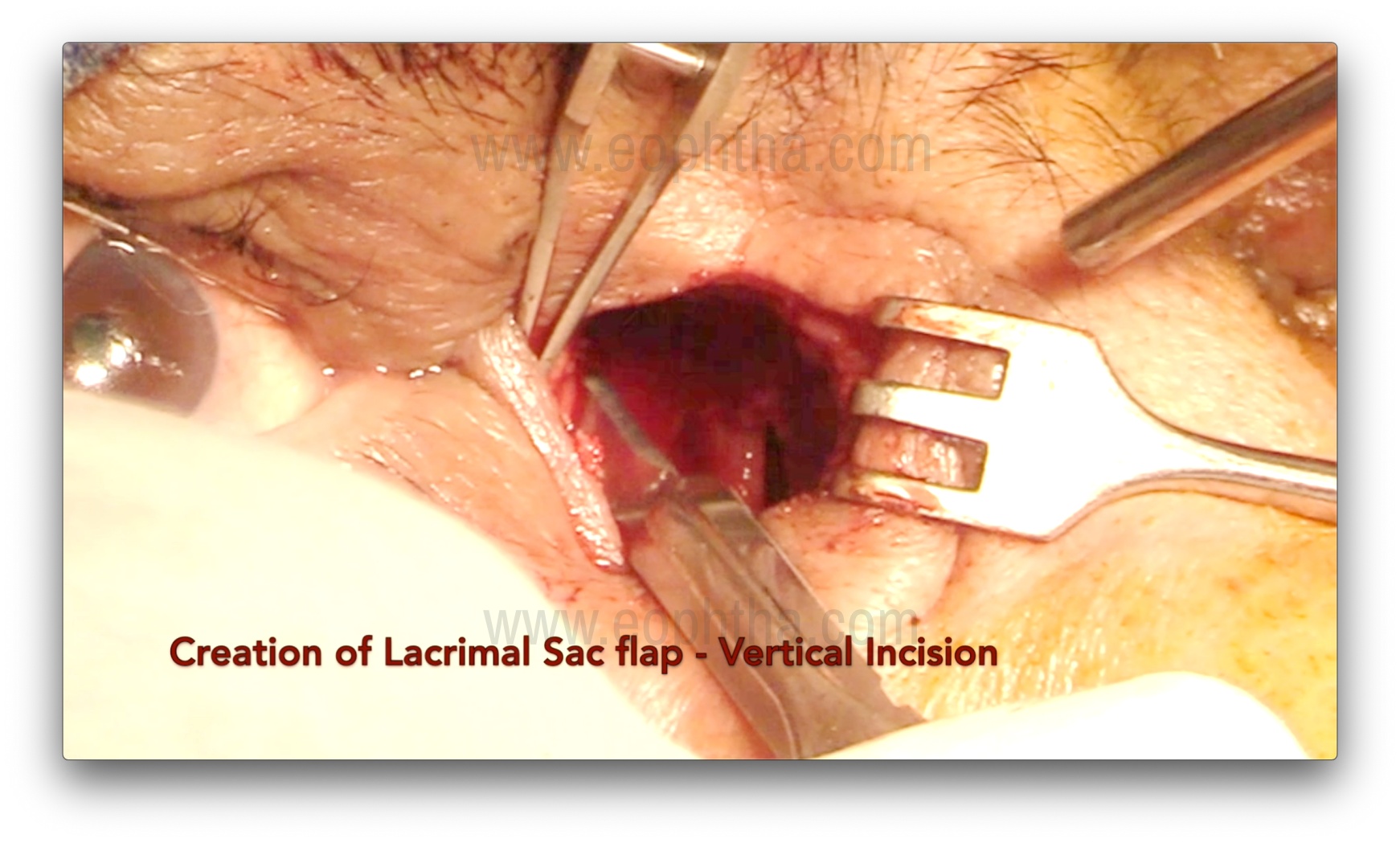
Lacrimal sac probing and Flap creation (Figure 11 & 12)
After adequate osteotomy, the upper punctum is dilated with a Nettleship punctal dilator, and a Bowman's probe is passed to tent the medial wall of the lacrimal sac. The sac may also be inflated with fluorescein-stained viscoelastic to facilitate dilation and ease the creation of the flap. A No. 15 blade is used to make a vertical incision from the fundus to the sac-duct junction, and then Westcott scissors are used to create either H- or U-shaped flaps. The anterior flap should be kept slightly longer. The posterior flap can be left as is and kept flat over the wall of the anterior ethmoid air cell. It is important to open the entire lacrimal sac in a book-like fashion.
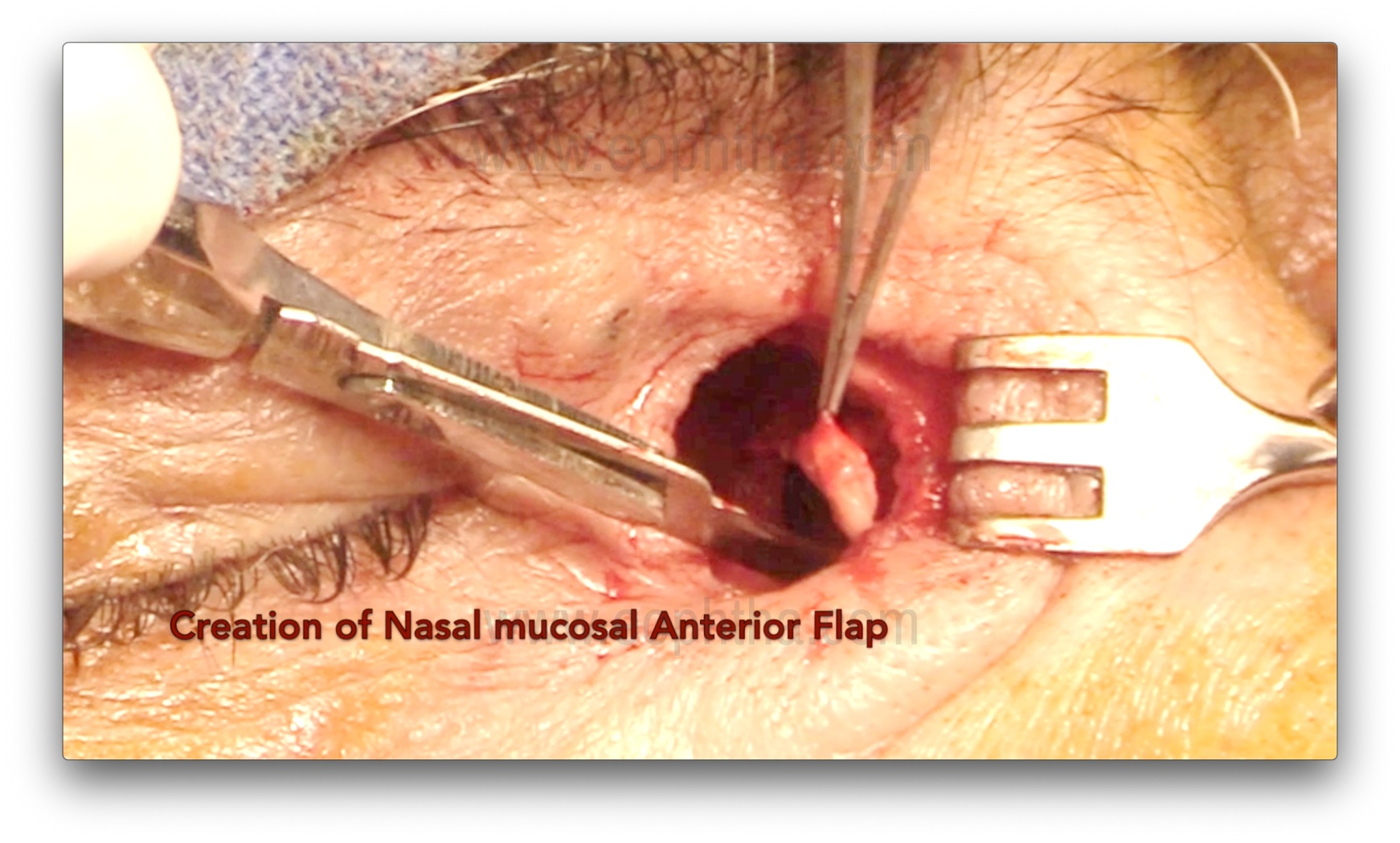
Nasal mucosal flap creation (Figure 13)
A No. 15 blade is used to first incise the nasal mucosa inferiorly (as the septum is farther from the lateral wall of the nose inferiorly), and then Westcott scissors are used to create either an H- or U-shaped flap. During this step, it is important to avoid injuring the septal mucosa, as it can form adhesions with the ostium and cause failures.
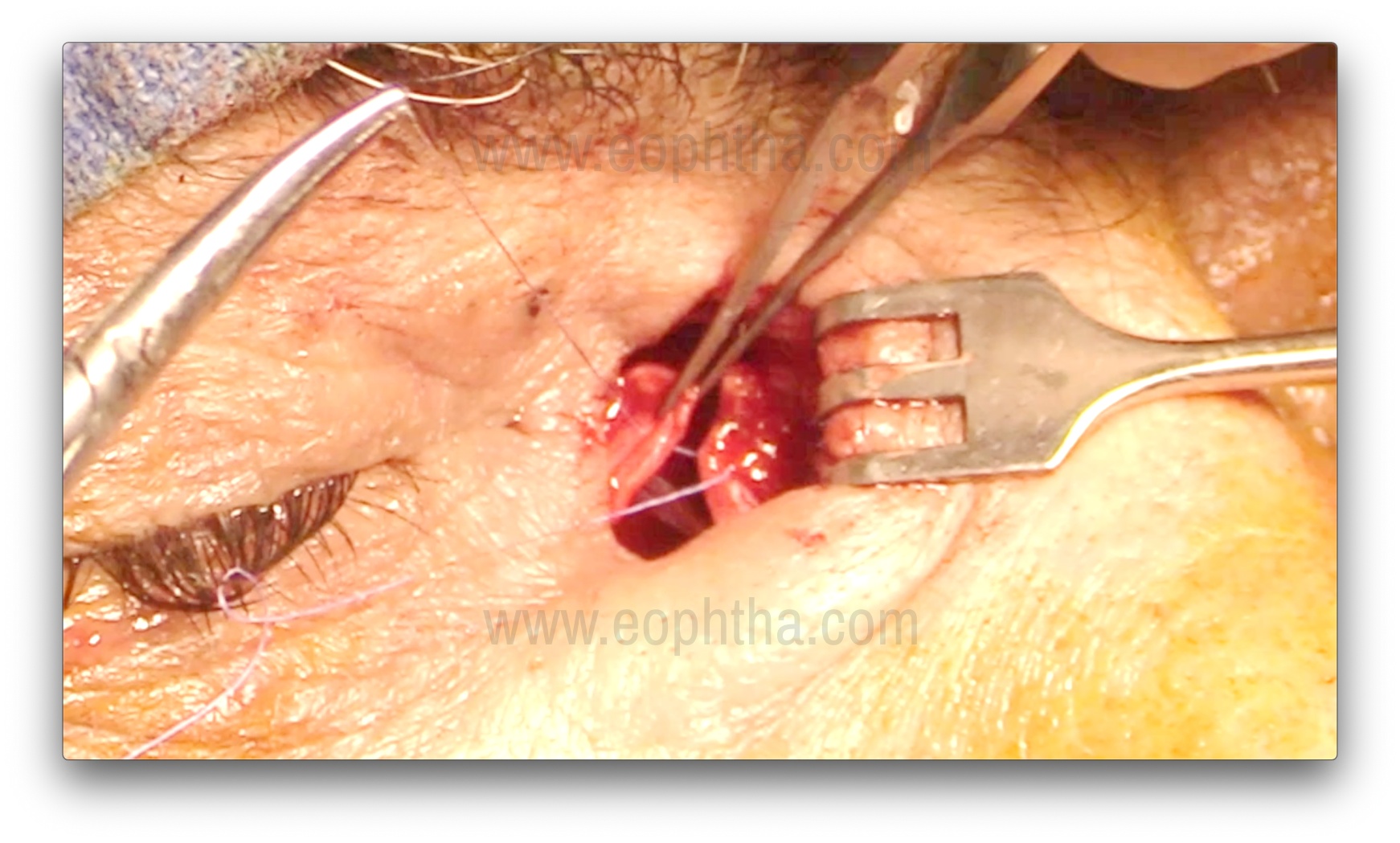
Suturing of Lacrimal and Nasal mucosal flaps (Figure 14)
Three 6-0 Vicryl interrupted sutures are placed, taking full-thickness mucosal bites through both the anterior flaps of the lacrimal sac and the nasal mucosa. Trimming of the anterior flaps may be performed if the flaps are too long, to avoid the sagging of flaps posteriorly. The flaps should be kept slightly taut, and this can be achieved with a suture between the flap and the periosteum over the lacrimal crest. Posterior flaps (formed with an H-shaped incision) can be left alone, excised, or sutured without any difference in the surgical success rate
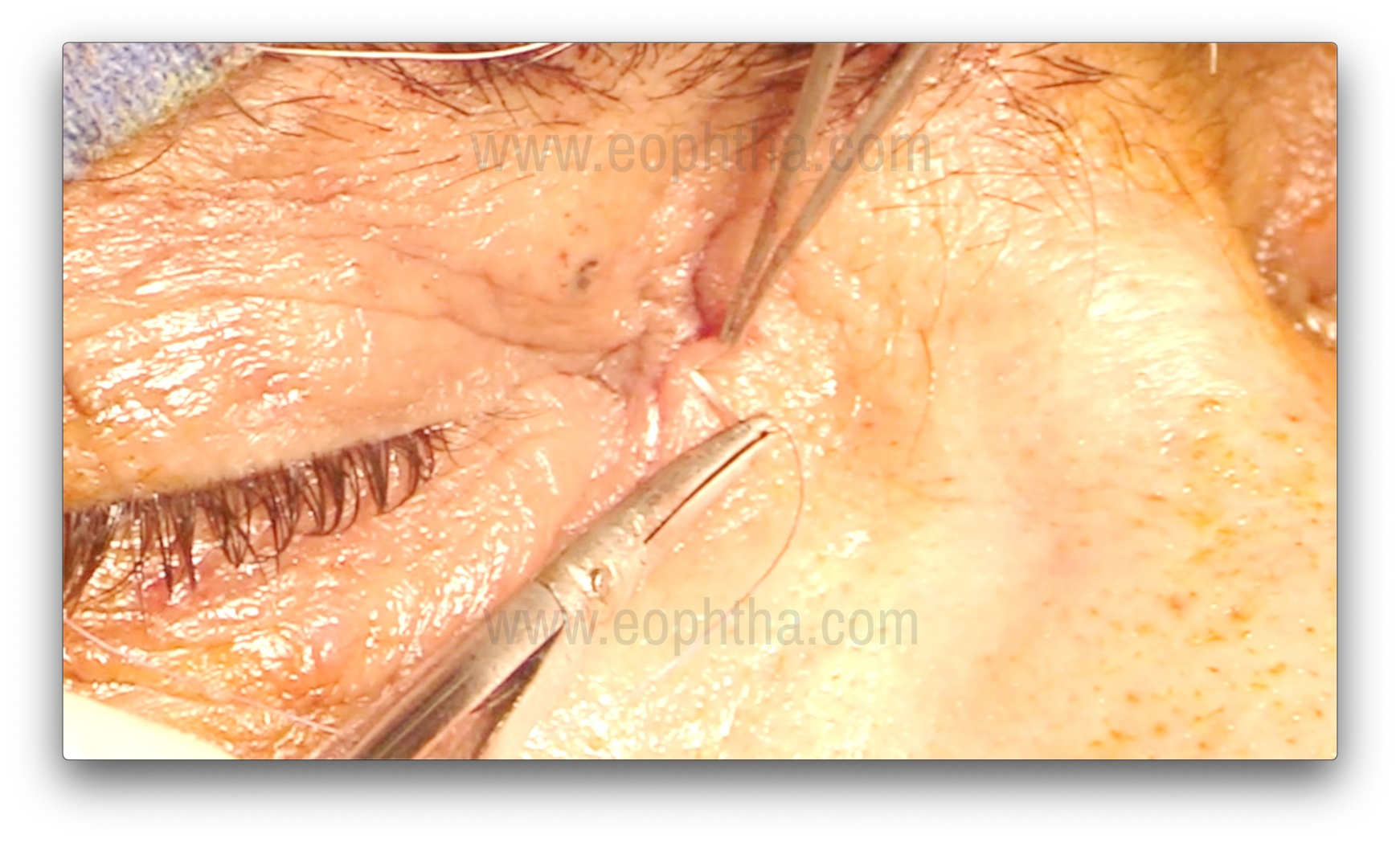
Closure of Orbicularis and Skin (Figure 15)
The orbicularis is closed with 3-4 interrupted 6-0 Vicryl sutures. Approximating the orbicularis keeps the wound edges vascularized, ensuring proper healing. The medial canthal tendon does not need to be resutured. The overlying skin is closed with 6-8 interrupted 6-0 Silk, Prolene, or Vicryl sutures.
The nasal pack is replaced with gauze soaked in betadine and antibiotic ointment. Antibiotic ointment is applied over the sutures, and a pressure bandage is applied.
Steps to achieve haemostasis
- Preoperative nasal decongestion with oxymetazoline
- Strict control of hypertension
- Cessation of anti-coagulants before surgery
- Use of Adrenaline mixed lignocaine in suitable patients
- Use of hypotensive anaesthesia during General anaesthesia
- Radio-frequency monopolar cautery
- Good working suction machine and cannula
- Good light source and operating bin-ocular magnification loupe or microscope (as per surgeon preference)
- Bone wax, absorbable surgicel, gel foam if needed
Post-operative care
Systemic oral antibiotics and NSAIDs are given for 5-7 days. Wound cleaning with a 5% betadine solution and antibiotic ointment application is advised twice a day for 2 weeks on the sutures. Antibiotic-steroid eye drops are prescribed for 4 weeks, although some surgeons prefer only antibiotic drops. Nasal decongestants are given for 3-4 weeks. The nasal pack and eye patch are removed the next day, and attention should be paid to any epistaxis. In the case of epistaxis, nasal packing is repeated and maintained for 48-72 hours. Skin sutures are removed 7-10 days post-surgery.
Use of Adjunctives during DCR
Bicanalicular silicone intubation and the application of Mitomycin-C (MMC) are the two most commonly used adjuncts to increase the success rate in selected cases. Indications for bicanalicular silicone intubation include previously failed DCR, a history of acute dacryocystitis/lacrimal abscess, the presence of canalicular stenosis, common canalicular block, a small fibrosed sac, small lacrimal and nasal mucosal flaps, extensive bleeding during surgery, and NLDO secondary to trauma. The indications for MMC are almost the same as for intubation, except for canalicular stenosis.
Complications of DCR Surgery
- Post-DCR Hemorrhage: Apart from intraoperative bleeding, immediate postoperative bleeding can occur within 24 hours, usually due to blood pressure normalization or increase after surgery and clot retraction. Delayed bleeding, occurring between 4-7 days as frank epistaxis, is typically due to clot retraction and friable blood vessels, and may require repeat nasal packing, monitoring of vitals, and antibiotic prophylaxis. Packing can be done with a Gelfoam-thrombin pack and should be maintained for 48 hours. In recalcitrant cases, embolization of the internal maxillary artery may be performed by an interventional radiologist.
- Orbital Emphysema: This occurs due to an opening into the ethmoid air cells during surgery, precipitated by forced nose blowing, leading to air collection in the preseptal subcutaneous tissues of the eyelid. Diagnosis is confirmed by characteristic crepitus on palpation. Management is conservative, but vision-threatening cases require needle aspiration of air.
- Orbital Hemorrhage: The source of bleeding is typically the anterior ethmoidal artery, septum openings, and injury to orbital fat. Clinical signs include proptosis, ecchymosis, limitation of ocular movements, and relative afferent pupillary defect. Urgent canthotomy and cantholysis may be necessary to relieve orbital pressure.
- Cerebrospinal Fluid (CSF) Leakage: Damage to the cribriform plate during osteotomy can cause CSF leakage and rhinorrhea. The cribriform plate, located approximately 15 mm above the common canaliculus, rarely gets damaged. Low-lying cribriform plate and post-traumatic conditions predispose to CSF leakage. Management includes nasal packing, with larger defects requiring repair using mucous membrane, fascia lata, temporalis fascia, or muscle.
- Infection and Gaping of Wound: Though rare, this can occur with poor wound hygiene or improper closure of the orbicularis and skin during surgery. Treatment includes removal of infected sutures, wound debridement, and resuturing.
- Scar Disfigurement: A hypertrophic scar is a rare complication. Management options include massage over the sac for 3 months with ointment, warm compresses, intralesional steroid injections, and surgical revision with V-Y plasty for an unsightly epicanthic fold.
- Persistent Epiphora: Causes include partial or complete closure of anastomosis, obstruction of the common canaliculus, granulation at the osteotomy site, bone regrowth, improper flap creation, drainage into the ethmoids, and lacrimal dysfunction. Sump syndrome, which occurs when the lacrimal sac is only opened superiorly, creates a reservoir for tears and debris collection, leading to regurgitation through the common canaliculus. This requires enlargement of the ostium and complete marsupialization of the sac.
- Intubation-Related Complications: These include punctal cheese wiring, migration or prolapse of the tube in front of the cornea, canalicular slitting, discharge, and infection around the tube. Management involves tube repositioning using an endoscope if sufficient time has not elapsed after surgery, or removal of the tube if almost sufficient time has elapsed.
- Mitomycin-C Related Complications: Commonly used concentrations are 0.2 mg/ml for 5 minutes to 0.5 mg/ml for minutes, with no statistical difference. One study noted 0.4 mg/ml for 2 minutes to be effective. In an experimental study, Ali et al. found the minimum effective concentration to be 0.2 mg/ml for 3 minutes. Toxic effects on cells were observed with 0.4 mg/ml beyond 5 minutes and at 0.5 mg/ml concentration regardless of application time, suggesting that higher concentrations may be harmful. Clinical complications related to MMC, such as mucosal necrosis, burns, infection, and bleeding, are very rare and not directly attributable to MMC use due to multiple confounding factors.
References:
- Toti A. Dacryocistorhinostomia. Clin Mod 1904;X:33-34.
- Caldwell GW. Two new operations for obstruction of the nasal duct, with preservation of the canaliculi and an incidental description of a new lacrimal probe.N Y Med J.1893;57:581–582.
- Massaro BM, Gonnering RS, Harris GJ. Endonasal laser dacryocystorhinostomy. A new approach to nasolacrimal duct obstruction.Arch Ophthalmol. 1990;108(8):1172-1176.
- Gonnering RS, Lyon DB, Fisher JC. Endoscopic laser-assisted lacrimal surgery.Am J Ophthalmol. 1991;111(2):152-157.
- Tutton MK, O'Donnell NP. Endonasal laser dacryocystorhinostomy under direct vision.Eye (Lond). 1995;9 ( Pt 4):485-487.
- Woog JJ, Metson R, Puliafito CA. Holmium:YAG endonasal laser dacryocystorhinostomy.Am J Ophthalmol. 1993;116(1):1-10.
- Metson R. Endoscopic surgery for lacrimal obstruction.Otolaryngol Head Neck Surg. 1991;104(4):473-479.
- Javate RM, Campomanes BS Jr, Co ND, et al. The endoscope and the radiofrequency unit in DCR surgery.Ophthalmic Plast Reconstr Surg. 1995;11(1):54-58.
- Tsirbas A, Wormald PJ. Endonasal dacryocystorhinostomy with mucosal flaps.Am J Ophthalmol. 2003;135(1):76-83.
- Sobel RK, Aakalu VK, Wladis EJ, Bilyk JR, Yen MT, Mawn LA. A Comparison of Endonasal Dacryocystorhinostomy and External Dacryocystorhinostomy: A Report by the American Academy of Ophthalmology.Ophthalmology. 2019;126(11):1580-1585.
- Karim R, Ghabrial, Lynch, Tang. A comparison of external and endoscopic endonasal dacryocystorhinostomy for acquired nasolacrimal duct obstruction.Clin Ophthalmol. 2011;5:979-989
- Ali MJ, Psaltis AJ, Murphy J, Wormald PJ. Powered endoscopic dacryocystorhinostomy: a decade of experience.Ophthalmic Plast Reconstr Surg. 2015;31(3):219-221.
- Ali MJ, Psaltis AJ, Bassiouni A, Wormald PJ. Long-term outcomes in primary powered endoscopic dacryocystorhinostomy.Br J Ophthalmol. 2014;98(12):1678-1680.
- Ali MJ, Naik MN, Honavar SG. External dacryocystorhinostomy: Tips and tricks.Oman J Ophthalmol. 2012;5(3):191-195.
- Hurwitz JJ. In: The Lacrimal System. Dacryocystorhinostomy. 1996, Lipincott-Raven. Philadelphia p 245-6.
- You YA, Fang CT. Intraoperative mitomycin C in dacryocystorhinostomy. Ophthal Plast Reconstr Surg 2001;17:115-9.
- Deka A, Bhattacharjee K, Bhuyan SK, Barua CK, Bhattacharjee H, Khaund G. Effect of mitomycin C on ostium in dacryocystorhinostomy. Clin Experiment Ophthalmol 2006;34:557-61.
- Ali MJ, Mariappan I, Maddileti S, Ali MH, Naik MN. Mitomycin C in dacryocystorhinostomy: The search for the right concentration and duration - A fundamental study on human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg 2013;29:469-74.
- Kamal S, Ali MJ, Naik MN. Circumostial injection of mitomycin C (COS-MMC) in external and endoscopic dacryocystorhinostomy: efficacy, safety profile, and outcomes. Ophthalmic Plast Reconstr Surg. 2014 Mar-Apr;30(2):187-90.
