Human immunodeficiency virus (HIV) disease is a pandemic of global concern, not only due to serious health issues affecting all organs of the body but also due to its huge economic burden.(1) The diagnosis is often associated with social stigma that includes changing sexual practices, lack of availability of a vaccine and the chronicity of the disease requiring lifelong treatment.(2) The disease runs a severe course, involving multiple organs. Ocular lesions are varied and can affect any structure, usually in the late phase of HIV infection. Rarely, these lesions can be the presenting manifestations of the disease.
While a variety of cell types may be infected by HIV, the immunologic hallmark is the selective loss of the CD4+ T cells. CD4 is a cell surface marker that identifies the helper/inducer subset of T cells. During the initial phases of infection, a majority of patients remain asymptomatic. With a decrease in the CD4+ T cell count (< 500 cells/µL) the immune system shows evidence of impairment, in the form of mild symptoms, such as fever, loss of weight and thrush. Further reduction in the CD4+ T cell count (< 200 cells/μL) leads to significant impairment of the immune system, characterized by a series of opportunistic infections or unusual neoplasms.(3) This chapter aims to review the characteristic ocular manifestations in HIV and briefly describe the phenomenon of immune recovery uveitis (IRU) in the highly active antiretroviral therapy (HAART) era.
|
HIV-associated ocular tumours |
Kaposi Sarcoma Ocular surface squamous neoplasia (OSSN) Ocular adnexal lymphoma and intraocular lymphoma |
|
HIV-associated ocular lesions |
Microangiopathy Uveitis Opportunistic infections |
Although transmission of the infection through ocular fluids such as tears (4) is not established, it is important to know that transmission can occur through corneal transplantation2 or contact with other intraocular fluids such as aqueous or vitreous fluids. (5) Morbidity and quality of life (QOL) are indicators of rehabilitation and are altered irreversibly if the ocular lesions are not detected early and appropriately managed. HAART has reduced AIDS-related mortality, but insufficient or improper management can affect the rehabilitation process.
Ocular lesions In AIDS:
Ophthalmic manifestations of AIDS were described as early as 1982 in the United States.(1) In India, these lesions were first reported in 1995, and later in 2000.(6,7) In this chapter, the ocular lesions will be described under the following headings:
- Ocular adnexal
- Anterior segment
- Posterior segment and neuroophthalmic
- Neoplasms
- Drug-induced (Iatrogenic)
- Immune Recovery Uveiits (IRU)
The ocular lesions in AIDS in the HAART era have varied manifestations and are marked by atypical presentations of known diseases. The immune status of a patient with HIV is determined by the CD4 T cell counts, and various studies demonstrate that a definite pattern of ocular complications is associated with various levels of CD4 cell counts (Table 1) (8)
|
CD4 counts (cells/ mm3) |
|
|
1000 |
Normal |
|
<500 |
Kaposi sarcoma Lumphoma Tuberculosis |
|
<250 |
Pneumocytosis Toxoplasmosis Retinal/ conjunctival microvasculopathy |
|
<100 |
Keratoconjunctivitis sicca VZV retinitis CMV retinitis |
Table 1 – Pattern of ocular complications with various levels of CD4 counts
Anterior segment lesions are seen in more than half of all HIV-infected patients, and almost one-third have ocular adnexal complications (Table 2) (9), which can be a sign of severe systemic immunosuppression. The anterior and posterior segment manifestations of HIV are listed in Tables 3 and 4 respectively.
|
Ocular adnexal manifestations |
Clinical characteristics |
|
Preseptal cellulitis |
Caused by Staphylococcus aureus, a commensal in the nasal mucosa Results in systemic and cutaneous infections |
|
Bacillary angiomatosis |
Caused by Bartonella Occurs when CD 4 counts ≤ 200 cells/µL Similar to Kaposi sarcoma |
Table 2: Ocular adnexal manifestations of opportunistic infections
Anterior segment manifestations |
Clinical Characteristics |
|
Herpes zoster ophthalmicus (HZO) |
Incidence: 10% - 20% of HIV positive patients (10,11) Risk of HZO: 6.6 times higher Characteristic feature: vesiculobullous rash along withthe distribution of the ophthalmic branch of the trigeminal nerve with necrotic skin lesions Signs: dendriform and stromal keratitis, conjunctivitis, blepharitis, uveitis (often with secondary glaucoma), hemorrhagic hypopyon, scleritis, retinitis, and encephalitis. Diagnosis: mainly clinical, viral culture, Tzanck smear, and polymerase chain reaction (PCR) |
|
Molluscum contagiosum |
Causative organism: large DNA pox virus Incidence: 5% - 18% of HIV infected patients (11,12) Pathogenesis: profound dysfunction of T lymphocyte mediatedlymphocyte-mediatedimmune response Characteristic feature: pink or pearly white wart-like umbilicated nodules on the skin (Figure 1) Aggressive presentation in children and young adults, involving the eyelid and conjunctiva, larger in number and size, often confluent, bilateral, and resistant to therapy Diagnosis: clinical examination of the lash line (13) Management: HAART, topical phenol, liquid nitrogen, lesion excision and cryotherapy |
|
Bacterial and viral keratitis |
Bacterial keratitis Causative organisms: Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa, Klebsiella oxytoca, Streptococcus, Bacillus, Micrococcus, Capnocytophaga and Acanthamoeba species (10,11) Characteristic feature: bilateral corneal ulcerations, polymicrobial and with a higher risk of perforation Microsporidial keratitis Presentation: bilateral superficial punctate epithelial keratitis, white intraepithelial infiltrates and follicular conjunctivitis with minimal anterior chamber reaction Diagnosis: Masson trichrome or Giemsa stain from conjunctival scrapings or corneal biopsy Treatment: fumagillin eye drops and oral albendazole are effective Viral keratitis Causative organisms: Varicella zoster virus (VZV), Herpes simplex virus (HSV), and Cytomegalovirus (CMV) Presentation: corneal scarring, iritis and raised intraocular pressure (14) |
Table 3: Anterior segment manifestations of opportunistic infections
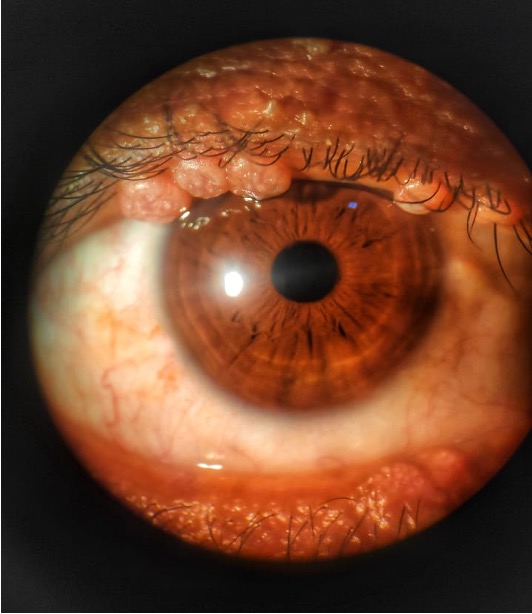
Figure1: Pink or pearly white wart-like umbilicated nodules on the skin of upper and lower eyelids (Photo courtesy: Dr. Rohit Rao)
| Posterior segment manifestations | Clinical characteristics |
| Cytomegalovirus (CMV) retinitis |
|
| Acute retinal necrosis (ARN) |
|
| Progressive outer retinal necrosis (PORN) |
|
| Ocular toxoplasmosis |
|
| Ocular tuberculosis (OTB) |
|
| Ocular syphilis |
|
| Neuro-ophthalmic manifestations |
|
Table 4: Posterior segment manifestations of opportunistic infections
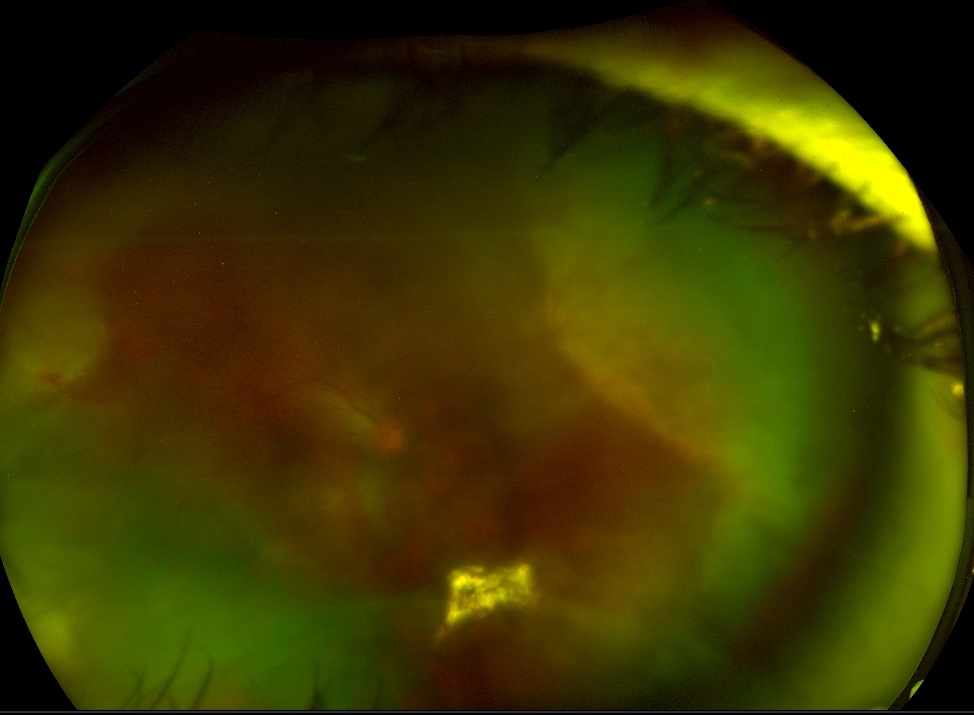
Figure 2: Fundus photograph of Acute Retinal Necrosis in a patient with HIV
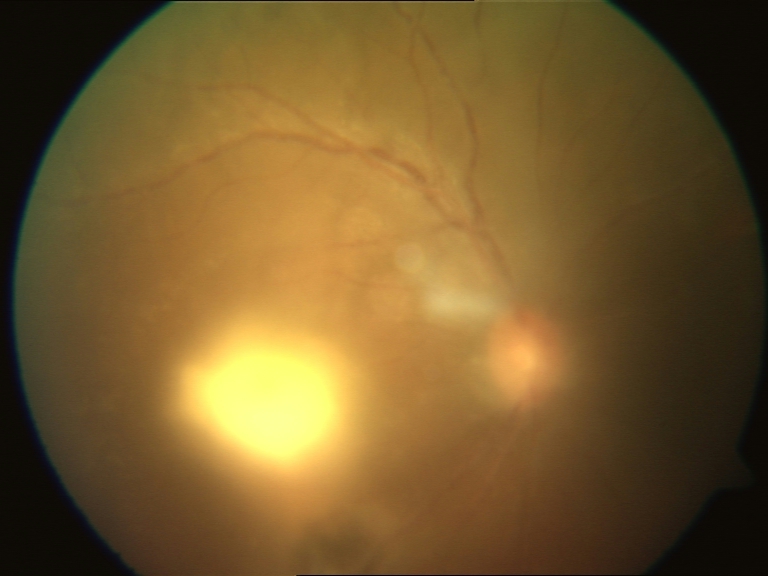
Figure 3: Ocular toxoplasmosis in HIV
The treatment of the above described anterior and posterior segment opportunistic infections is outlined in table 5.
|
Opportunistic infection |
Treatment of choice |
Alternate therapy |
Adverse effects |
|
|
HZO |
Oral acyclovir 800mg five times a day or oral valacyclovir 1g thrice daily |
Oral famciclovir or foscarnet- in resistant cases |
Nephrotoxicity |
|
|
Viral Keratitis |
Topical therapy VZV and HSV: Acyclovir eye ointment five times daily CMV: Ganciclovir gel 0.15% five times daily until healing occurs followed by |
Vidarabine 3% ointment five times daily or Famciclovir (125-500 mg three times daily) |
Nephrotoxicity, Myelosuppression |
|
|
CMV retinitis |
Ganciclovir (IV) |
Foscarnet (IV) induction: 5 mg/ kg weekly for 3 weeks Cidofovir induction: |
Ganciclovir/ valganciclovir: myelosuppression |
|
|
Ocular toxoplasmosis |
Pyrimethamine: 200 mg on the first day followed by 75- 100 mg daily |
Atovaquone, azithromycin 500 mg followed by 250 mg once a day |
Myelosuppression,leukopenia,pseudomembranous colitis |
|
|
Ocular TB |
H - isoniazid R - rifampicin Z - pyrazi- namide E - ethambutol S - streptomycin New case (never had treatment or less than 1 month of ATT): 2 HRZE + 4 HRE |
Multidrug resistant (MDR) TB: |
Hepatotoxicity, peripheral neuropathy, optic neuropathy |
|
|
Ocular syphilis |
Aqueous crystalline peni- cillin G: 18-24 million units/ day administered as 3-4 mil- lion units IV every 4 hours or continuous infusion for |
Procaine penicillin G: 2.4 mil- |
Jarisch-Herxheimer reaction |
|
|
ARN/ PORN |
Acyclovir induction: intra- venous acyclovir 500 mg every 8 hours for 2 to 3 weeks |
Nephrotoxicity |
Table 5 : Recommended treatment guidelines for various opportunistic infections
Neoplasms
(i) Kaposi sarcoma
It is a highly vascular mesenchymal tumor characterized by the presence of multiple purple‐to‐red nodules on the skin and mucous membrane. It is caused by human herpesvirus-8 (HHV‐8) or KS herpesvirus (KSHV), and occurs when CD4+ cells are ≤150 cells/μL.(34) Approximately, 10% to 20% of HIV-infected individuals are affected with Kaposi sarcoma (KS), involving the eyelids, conjunctiva, and, rarely, the orbit. The tumor is relatively rare in India. When present on the lids, the tumour can mimic a chalazion, while in the conjunctiva it can be mistaken for a subconjunctival hemorrhage or a pyogenic granuloma. For the purpose of prognosis, the tumor is staged as follows:
- Stages I and II lesions are flat (less than 3 mm in height), patchy, and of up to 4 months duration
- Stage III lesions are nodular (greater than 3 mm in height) and greater than 4 months duration
- Accurate staging determines response to therapy.(35)
Treatment depends on the site and extension of KS and includes radiotherapy, cryotherapy, surgical excision and systemic chemotherapy. Localized lesions can be surgically excised with or without adjunctive cryotherapy to the margins. Systemic agents are warranted for patients with advanced disease, such as extensive cutaneous disease, visceral disease, or lymphedema. HAART has been successful in treating AIDS-related primary KS and has reduced the need for other methods such as chemotherapy or damaging radiation to control the disease.(36) Chemotherapy with pegylated liposomal doxorubicin, paclitaxel, bleomycin, lenalidomide, interferon α, and immune checkpoint inhibitors (nivolumab, pembrolizumab) are under investigation.(37)
(ii) Ocular surface squamous neoplasia (OSSN)
It is a common primary malignancy of the eye. Risk factors include exposure to ultraviolet (UV) rays, human papillomavirus (HPV) infection, and chemicals such as arsenic and hydrocarbon. Increased incidence of OSSN is reported in HIV patientsand is a presenting sign in more than two-thirds of patients. In individuals with HIV, it can occur at an earlier age (3rd/4th decade) and affects both the cornea and the conjunctiva. (38) It may present with unilateralorbilateral, pigmentedor non‐pigmented papillary lesions with intra‐lesional blood vessel (feeder's vessel) and fronds, raised plaque with keratinization, or as a diffuse gelatinous form. Patients may be asymptomatic or have mild symptoms such as foreign body sensation, irritation, or mild pain. HIV‐infected patients have larger, more aggressive tumors, higher‐grade malignancy with a higher incidence of corneal, scleral, and orbital invasion. Differential diagnoses include pinguecula, pterygium, and papilloma. Diagnosis is by an incision or excision biopsy. Surgical treatment options include tumor excision with amniotic membrane grafting with adjunctive cryotherapy or chemotherapy; while medical management involves topical mitomycin‐C (MMC) or 5‐flurouracil (5-FU) for a week, followed by a drug holiday, and repetition of the cycle until regression ofthelesion. Monitoring for side effects such as epithelial toxicity is recommended. Since interferon is better tolerated, it can be administered either as eye drops or weekly subconjunctival injections. Recently, a possible role of HPV vaccination in the prevention of OSSN is under consideration.(38)
(iii) Ocular adnexal lymphoma and intraocular lymphomas
These are the B‐cell non‐Hodgkins lymphomas (NHL) seen in HIV/AIDS with a poor prognosis.(39) They appear as erythematous lesions affecting the orbit, lacrimal glands, conjunctivae,and eyelids. The management of low-grade tumors includes chemotherapy with chlorambucil or fludarabine and that of high-grade lymphomas include, CHOP (cyclophosphamide, vincristine, doxorubicin and prednisolone) or radiotherapy. Novel treatments under investigation are monoclonal antibodies targeted against CD40 and bortezomib, a proteasome inhibitor that targets the intracellular pathways involved in tumor cell survival and growth.(40)
Diagnosis and Management in the HAART era
One of the hallmarks of progressive immune deficiency is a steady decline in the absolute number of CD4+ T-lymphocytes.(7) In the pre-HAART era, the lifetime cumulative risk for developing at least one abnormal ocular lesion in those with HIV, ranged from 52% to 100%.(41) Treatment of HIV infection with the HAART regimen aims to inhibit progression to full-blown AIDS (defined by a CD4+ cell count of < 200 cells/μL), or death. This is achieved by reduction in plasma HIV RNA to permanently low levels, reduction in the viral load and an increase in CD4+ cell counts which improve the immune status of the individual. Those on HAART are less likely to be affected by blinding posterior segment infections, and there has even been a marked reduction in the occurrence of anterior-segment and adnexal lesions in patients who received treatment.
Patients with HIV/AIDS can develop ocular lesions from the drugs used for treatment of the immunocompromised state or from those used in the management of opportunistic infections.
HAART-induced immune recovery has its own merits and demerits, with 10% to 25% of patients experiencing immune recovery problems or immune reconstitution inflammatory syndrome (IRIS).(11,42)
Drug-induced Uveitis
Careful monitoring is advocated during treatment with HAART, especially in elderly patients due to the high degree of concurrent medication use and, the greater potential for harmful drug interactions, as well as age-related changes in renal and hepatic function that could affect drug clearance.(43) Similar complications may be seen in the eye, presenting as uveitis, following systemic and intraocular therapies. Most commonly implicated drugs are cidofovir and rifabutin. Cidofovir is a nucleotide analog that acts against CMV. It has been associated with side effects such as granulomatous anterior uveitis with both intravenous and intravitreal routes of administration.(44) The increased number of CD4+ T cells following HAART can lead to an inflammatory response resulting in uveitis.(45) Co‐administration of probenecid, a competitive inhibitor of organic anion transport in epithelial barriers reduces the chance of anterior uveitis by almost 50%. Rifabutin is commonly used in the treatment of pulmonary tuberculosis and for prophylaxis of Mycobacterium avium complex (MAC) in patients with AIDS and low CD4+ T‐cell counts. It is a less potent inducer of the cytochrome P450 drug metabolizing enzymes than rifampicin and is often used as a replacement drug. Its use can commonly cause hypopyon uveitis, intermediate uveitis or posterior uveitis.(45) The pathogenesis for uveitis is correlated to both direct chemical drug toxicity and immune complex deposition. While the long duration of treatment associated with the development of uveitis supports the immunological model of inflammation, short-term drug exposure would be expected to cause drug toxicity reaction.Other HAART drugs with ocular side effects include nevirapine, didanosine, ritonavir,indinavir, efavirenz and newer elvitegravir-based combination therapies.(45-49) Most cases of drug-induced uveitis respond well to topical corticosteroid and cycloplegic agents. Discontinuation of the inciting drug is required in refractory uveitis that does not respond to conventional treatment or in case of recurrences or other complications.(45)
Immune Recovery Uveitis
Before the introduction of protease inhibitors in the treatment of HIV, patients with CMV retinitis typically had CD4+ T lymphocyte counts <50 cells/μL, with minimal intraocular inflammation. Partial recovery of immune responses specific to residual CMV antigen located in the eye results in Immune recovery uveitis (IRU). It affects the anterior uvea and vitreous, and is associated with visual impairment. IRU is part of the immune reconstitution inflammatory syndrome (IRIS). These reactions may either worsen the clinical manifestations of the opportunistic infection (paradoxical reaction), or develop new manifestations shortly after the initiation of HAART (unmasking syndromes).(27)
The AIDS Clinical Trial group defined IRU as
- decrease in vision accompanied by at least 2 of the following signs in the absence of active CMV retinitis: presence of >2+ inflammatory cells in the vitreous; cystoid macular edema (CME); or epiretinal membrane (ERM) formation in patients receiving antiretroviral therapy with evidence of immune reconstitution.(50)
- Immune reconstitution, in turn, was defined as a CD4+ count of 100 cells/μL or more.
It is estimated that IRIS develops in 15-25% of HIV-positive patients receivingHAART, with the highest incidence during 8-16 weeks following the initiation oftreatment.(51,52) Therefore, to prevent the occurrence and severity of IRU, it may be advised to delay initiation of HAART, until CMV retinitis is managed with treatment. However, careful clinical judgment and close monitoring of the patient are optimal to prevent mortality.
IRU has varying clinical presentations. It may be symptomatic or asymptomatic, with a self-limiting or chronic inflammatory course. Visual loss or floaters arethe most common features of symptomatic IRU. IRU-induced vision loss is generallymoderate, which typically results from complications of the intraocular inflammation.However, occasionally it may result in severe visual loss (visual acuity 20/200 orworse).(53,54) The most common inflammatory complications of IRU are CME and ERM, followed by preretinal neovascularization, papillitis, proliferative vitreoretinopathy and anterior segment inflammation that leads to iris synechiae and cataract.(27,54,55)
A thorough clinical and ancillary examination must be carried out in patients withmanifestations of ocular inflammation undergoing HAART, to exclude co-infectionssuch as syphilis, drug toxicity or interactions, and primary manifestations of ocularopportunistic infections. PCR of intraocular fluids can be used as a confirmatory test.Aqueous or vitreous aspirate PCR may be used to rule out co-existing causes of uveitis,when available.(56) The CMV Pp65 antigenemia assay quantitates the number ofCMV-infected leukocytes in peripheral blood. It has proven efficacy in the detection and monitoring of CMV infection in immunocompromised patients.(57)
In cases of mild and more advanced IRU, topical or periocular corticosteroids may be sufficient to control inflammation and CME.(27) The use of intravitreal triamcinolone acetonide (IVTA) injections has been reported.(58, 59) Long-term intravitreal implanted corticosteroid depot has been proven effective.(60) Oral valganciclovir has been observed to show a reduction in CME following treatment.(61)
Tips in the management of a patient with suspected ocular HIV
Do’s (when to suspect and test for HIV)
- Younger patients (<45 years) with HZO or typical ocular manifestations
- Corneal perforation in a patient with HZO infection
- Molluscum lesions in a young adult
- Atypical presentations of ocular disease
Don’t’s (pertaining to careful systemic and treatment history)
- Overlook early HIV microangiopathy
- Treat IRU with aggressive steroid therapy without monitoring for signs of CMV retinopathy (CMVR) reactivation
- Consider all CMVR to be HIV-related. Rule out other causes of immunosuppression, organ transplantation, diabetes mellitus, local steroid therapy (intra/ periocular)
- Overlook patient’s CD4+ counts and HIV treatment regime
Conclusion
Ocular complications of HIV are numerous and can be sight-threatening. The presence of multiple but distinct pathogens from the eye provides a definitive clue to HIV-related systemic disorders. HAART has remarkably reduced the frequency of rare malignancies and severe immunosuppression. Patients on HAART can develop severe inflammatory reaction due to the immune recovery phenomenon, which in itself may be a source of severe ocular morbidity.
Patient awareness about the signs and symptoms of ocular complications of HIV is an important part of their management. Routine monitoring is also an essential tool in the management of these patients, including those with immune
References
1. Holland GN, Gottlieb MS, Yee RD, Schanker HM, Pettit TH. Ocular disorders associated with a new severe acquired cellular immunodeficiency syndrome. Am J Ophthalmol 1982;93:393‑402. 2. Joshi RK, Mehendale SM. Determinants of consistently high HIV prevalence in Indian Districts: A multi‑level analysis. PLoS One 2019;14:e0216321. 3. Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623-83. 4. Ablashi DV, Sturzenegger S, Hunter EA, et al. Presence of HTLV-III in tears and cells from the eyes of AIDS patients. J Exp Pathol 1987;3:693–703. 5. Jabs DA, Green WR, Fox R, Polk BF, Bartlett JG. Ocular manifestations of acquired immune deficiency syndrome. Ophthalmology 1989;96:1092–1099. 6. Biswas J, Madhavan H N and Badrinath SS: Ocular lesions in AIDS: A report of first two cases in India. Indian J Ophthalmol 1995;43: 69–72. 7. Biswas J, Madhavan HN, George AE, Kumarasamy N, Solomon S. Ocular lesions associated with HIV infection in India: A series of 100 consecutive patients evaluated at a referral center. Am J Ophthalmol 2000; 129(1): 9–15. 8. Biswas J, Sudharshan S. Ophthalmic manifestations of HIV/AIDS. Hall, Hall and Cockerell : HIV/AIDS in the post-HAART era . Chapter 14. p 293-318. 9. Nema HV, Nema N. Recent Advances in Ophthalmology-12. JP Medical Ltd; 2015:127-151. 10. Jeng BH, Holland GN, Lowder CY, Deegan WF 3rd, Raizman MB, Meisler DM. Anterior segment and external ocular disorders associated with human immunodeficiency virus disease. Surv Ophthalmol. 2007;52(4):329‐68. 11. Biswas J, Sudharshan S. Anterior segment manifestations of human immunodeficiency virus/acquired immune deficiency syndrome. Indian J Ophthalmol. 2008;56(5):363‐75. 12. Meza‐Romero R, Navarrete‐Dechent C, Downey C. Molluscum contagiosum: An update and review of new perspectives in etiology, diagnosis, and treatment. Clin Cosmet Investig Dermatol. 2019;12:373‐81. 13. Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147(6):652‐4. 14. Azher TN, Yin XT, Tajfirouz D, Huang AJ, Stuart PM. Herpes simplex keratitis: Challenges in diagnosis and clinical management. Clin Ophthalmol. 2017;11:185‐91. 15. Accorinti M, Pirraglia MP, Corradi R, Corsi C, Fabiani C, Pivetti‐Pezzi P. Changing patterns of ocular manifestations in HIV seropositive patients treated with HAART. Eur J Ophthalmol. 2006;16(5):728‐32. 16. Sudharshan S, Kaleemunnisha S, Banu AA, et al. Ocular lesions in 1,000 consecutive HIV‐positive patients in India: A long‐term study. J Ophthalmic Inflamm Infect. 2013;3(1):2. 17. Munro M, Yadavalli T, Fonteh C, Arfeen S, Lobo‐Chan AM. Cytomegalovirus retinitis in HIV and non‐HIV individuals. Microorganisms 2019;8(1):55. 18. Jabs DA, Ahuja A, Van Na a ML, et al. Long‐term outcomes of cytomegalovirus retinitis in the era of modern antiretroviral therapy: Results from a United States Cohort. Ophthalmology. 2015;122(7):1452‐63. 19. Schoenberger SD, Kim SJ, Thorne JE, et al. Diagnosis and treatment of acute retinal necrosis: A report by the American Academy of Ophthalmology. Ophthalmology. 2017;124(3):382‐92. 20. Cunningham ET Jr, Wong RW, Takakura A, Downes KM, Zierhut M. Necrotizing herpetic retinitis. Ocul Immunol Inflamm. 2014;22(3):167‐9. 21. Meisheri YV, Mehta S, Patel U. A prospective study of seroprevalence of Toxoplasmosis in general population, and in HIV/AIDS patients in Bombay, India. J Postgrad Med. 1997;43(4):93‐7. 22. Moshfeghi DM, Dodds EM, Couto CA, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology. 2004;111(4):716‐25. 23. Mahalakshmi B, Therese KL, Madhavan HN, Biswas J. Diagnostic value of specific local antibody production and nucleic acid amplification technique‐nested polymerase chain reaction (nPCR) in clinically suspected ocular toxoplasmosis. Ocul Immunol Inflamm. 2006;14(2):105‐12. 24. Babu RB, Sudharshan S, Kumarasamy N, Therese L, Biswas J. Ocular tuberculosis in acquired immunodeficiency syndrome. Am J Ophthalmol. 2006;142(3):413‐8. 25. Rathinam SR, Lalitha P. Paradoxical worsening of ocular tuberculosis in HIV patients after antiretroviral therapy. Eye (Lond). 2007;21(5):667‐8. 26. Ganesh SK, Abraham S, Sudharshan S. Paradoxical reactions in ocular tuberculosis. J Ophthalmic Inflamm Infect. 2019;9(1):19. 27. Otiti‐Sengeri J, Meenken C, van den Horn GJ, Kempen JH. Ocular immune reconstitution inflammatory syndromes. Curr Opin HIV AIDS. 2008;3(4):432‐7. 28. Walker NF, Stek C, Wasserman S, Wilkinson RJ, Meintjes G. The tuberculosis‐associated immune reconstitution inflammatory syndrome: Recent advances in clinical and pathogenesis research. Curr Opin HIV AIDS. 2018;13(6):512‐21. 29. Mehta S, Mansoor H, Khan S, Saranchuk P, Isaakidis P. Ocular inflammatory disease and ocular tuberculosis in a cohort of patients co‐infected with HIV and multidrug‐resistant tuberculosis in Mumbai, India: A cross‐sectional study. BMC Infect Dis. 2013;13:225. 30. Lee SY, Cheng V, Rodger D, Rao N. Clinical and laboratory characteristics of ocular syphilis: A new face in the era of HIV co‐infection. J Ophthalmic Inflamm Infect. 2015;5(1):56. 31. Workowski KA, Bolan GA. Centers for disease control and prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137. Erratum in: MMWR Recomm Rep. 2015;64(33):924. 32. Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22(2):137‐47. 33. Jeyaraman VA, Sudharshan S, Selvakumar A, et al. Isolated cortical blindness without simultaneous neurological involvement in progressive multifocal leukoencephalopathy in a patient with human immune deficiency virus infection. J Ophthalmic Inflamm Infect. 2013;3(1):3. 34. Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers 2019;5(1):9. 35. Dugel PU, Gill PS, Frangieh GT, Rao NA. Treatment of ocular adnexal Kaposi’s sarcoma in acquired immune deficiency syndrome. Ophthalmology 1992;99:1127–1132. 36. International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 2000;92:1823–1830. 37. Dupin N. Update on oncogenesis and therapy for Kaposi sarcoma. Curr Opin Oncol. 2020;32(2):122‐8. 38. Rathi SG, Ganguly Kapoor A, Kaliki S. Ocular surface squamous neoplasia in HIV‐infected patients: Current perspectives. HIV AIDS (Auckl). 2018;10:33‐45. 39. Ferry JA, Fung CY, Zukerberg L, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol. 2007;31(2):170‐84. 40. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867‐74. 41. Hodge WG, Seif SR, Margolis TP. Ocular opportunistic infection incidences among patients who are HIV positive compared to patients who are HIV negative. Ophthalmology 1998;105:895–900. 42. French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS 2004;18:1615–1627. 43. Slim J, Saling CF. A Review of Management of Inflammation in the HIV Population. Biomed Res Int. 2016;2016:3420638. 44. London NJ, Garg SJ, Moorthy RS, Cunningham ET. Drug‐induced uveitis. J Ophthalmic Inflamm Infect. 2013;3(1):43. 45. Testi I, Agarwal A, Agrawal R, et al. Drug‐induced uveitis in HIV patients with ocular opportunistic infections. Ocul Immunol Inflamm. 2020;28(7):1069-75. 46. Dodi F, Alessandrini A, Camera M, Gaffuri L, Morandi N, Pagano G. Stevens‐Johnson syndrome in HIV patients treated with nevirapine: Two case reports. AIDS 2002;16(8):1197‐8. 47. Whitcup SM, Butler KM, Caruso R, et al. Retinal toxicity in human immunodeficiency virus‐infected children treated with 2',3'‐dideoxyinosine. Am J Ophthalmol. 1992;113(1):1‐7. 48. Sen P, Sudharshan S, Banerjee A, Dhami A. Clinical and electrophysiological characteristics of Efavirenz‐induced macular toxicity. GMS Ophthalmol Cases. 2020;10:Doc08. 49. Papavasileiou E, Younis S, Zygoura V, Quijano C, Jackson TL. Ritonavir‐associated toxicity mimicking retinitis pigmentosa in an HIV‐infected patient on highly active antiretroviral therapy. Retin Cases Brief Rep. 2017;11(4):306‐9. 50. Holland GN, Vaudaux JD, Shiramizu KM et al.; Southern California HIV/Eye Consortium. Characteristics of untreated AIDS-related cytomegalovirus retinitis. II. Findings in the era of highly active antiretroviral therapy (1997 to 2000). Am J Ophthalmol 2008;145(1):12–22. 51. French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active anti- retroviral therapy. HIV Med. 2000; 1(2):107-15. 52. Hirsch HH, Kaufmann G, Sendi P, Battegay M. Immune reconstitution in HIV infected patients. Clin Infect Dis. 2004;38(8):1159-66. 53. Robinson MR, Reed G, Csaky KG, Polis MA, Whitcup SM. Immune recovery uveitis in patients with cytomegalovirus retinitis taking highly active antiretroviral therapy. Am J Ophthalmol. 2000;130(1):49-56. 54. Thorne JE, Jabs DA, Kempen JH, et al. Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113(8):1423-40. 55. Kempen JH, Min YI, Freeman WR, et al. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006; 113(4):684-94. 56. Westeneg AC, Rothova A, De Boer JH, Groot-Mijnes JD. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann – Witmer coefficient in aqueous analysis. Am J Ophthalmol. 2007;144(5):781-5. 57. Ho SK, Lo CY, Cheng IK, Chan TM. Rapid cytomegalovirus pp65 antigenemia assay by direct erythrocyte lysis and immunofluorescence staining. J Clin Microbiol. 1998;36(3):638-40. 58. Morrison VL, Kozak I, Labree LD, et al. Intravitreal triamcinolone acetonide for the treatment of immune recovery uveitis macula oedema. Ophthalmology. 2007;114(2):334-9. 59. Sirimaharaj M, Robinson MR, Zhu M, et al. Intravitreal injection of triamcinolone acetonide for immune recovery uveitis. Retina. 2006;26(5):578–80. 60. Rothova A. Inflammatory cystoid macular oedema. Curr Opin Ophthalmol. 2007;18(6):487-92. 61. Kosobucki BR, Goldberg DE, Besshok, et al. Valganciclovir therapy for immune recovery uveitis complicated by macula oedema. Am J Ophthalmol. 2004;137(4):636-8.
