A 60-year-old-male presented to the Cornea clinic with diminution of vision in the left eye for 4 months which was associated with intermittent photophobia and colored haloes around lights, especially on waking up in the morning. The patient also complains of pain and watering.
No history of associated redness, itching, or discharge.
Past Ocular History:
H/o OD cataract surgery 6 years, OS cataract surgery 4 months back. No h/o trauma.
Past Medical History/ History of Medication:
No significant systemic history could be elicited. The patient was not on any medication. No addictions.
Family History:
No significant family history found
Review of Systems/Systemic Examination:
Systemic examination was within normal limits. All vitals were within normal limits.
OCULAR EXAMINATION
Best Corrected Visual Acuity (Snellen)
Right eye (OD): 6/9
Left eye (OS): CF 2 m
Ocular Motility/Alignment -Full, free, and painless in all gazes
Intraocular Pressure (IOP)
OD: 18 mm of Hg
OS: 14 mm of Hg
Pupils- OU Round, regular and reacting to light
Confrontation visual fields:CVF full
Slit-lamp examination:
|
OD |
OS |
|
|
Lids/lashes |
Normal |
Normal |
|
Conjunctiva/sclera |
Normal |
A 7 mm surgical incision scar visible at sclera above limbus superiorly from 10 to 2 o’clock |
|
Cornea |
Few pigments on endothelium |
Normal in size, shape, and curvature. Diffuse haze presents all over the cornea. Epithelium: Surface irregular with loss of luster Multiple small sub-epithelial micro and macro bullae involving whole cornea (largest measuring approx. 1.5 mm). Punctate stain positive along with areas of fluorescein pooling. No subepithelial fibrosis. (Fig. 1A) Stroma: Diffuse stromal edema and scarring present. (Fig. 1B) Descemet’s layer: Mild Descemet’s membrane thickening with folds. Endothelium: Diffuse pigment deposition on endothelium more so in pupillary area and in the nasal quadrant. No IOL endothelium contact. Corneal sensations are reduced in all quadrants. |
|
Anterior chamber |
Normal in depth, quiet |
Normal in depth, quiet |
|
Iris |
Normal color and pattern |
Normal color and pattern |
|
Lens |
PCIOL |
PCIOL |
Dilated fundus examination
|
OD |
OS |
|
|
Vitreous |
Clear |
Very hazy view due to corneal edema. USG Bscan done was within normal limits. |
|
Disc |
0.4:1, NRR healthy |
|
|
Macula |
FR dull |
|
|
Vessels |
Normal in calibre, AV ratio maintained |
|
|
Periphery |
Normal |
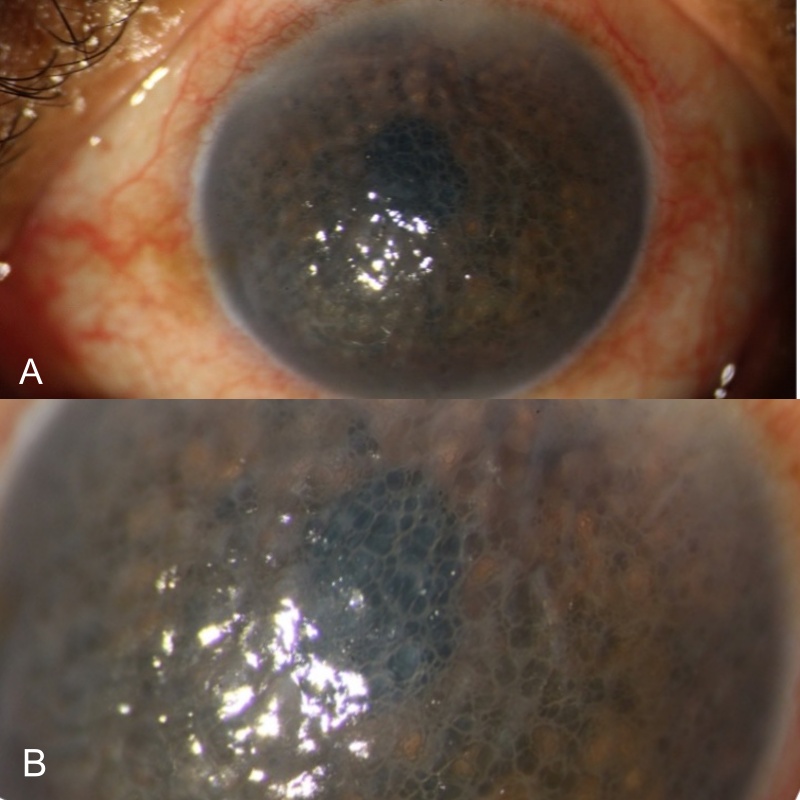
Figure1A & B – Shows multiple epithelial bullae and stromal edema
ANCILLARY INVESTIGATIONS:
Specular microscopic examination:
Preoperatively specular microscopy is indicated in suspected patients such as endothelial dystrophies or with a history of multiple previous surgeries. The mean cell area increases whereas mean cell density decreases in pseudophakic bullous keratopathy. (Fig 2A) Quantitative indices such as coefficient of variation(CV), percentages of hexagonality (6A) and mean cell area with standard deviation (SD) are abnormal depending on the extent of endothelial cell loss and the duration of corneal edema. (Fig. 2B)
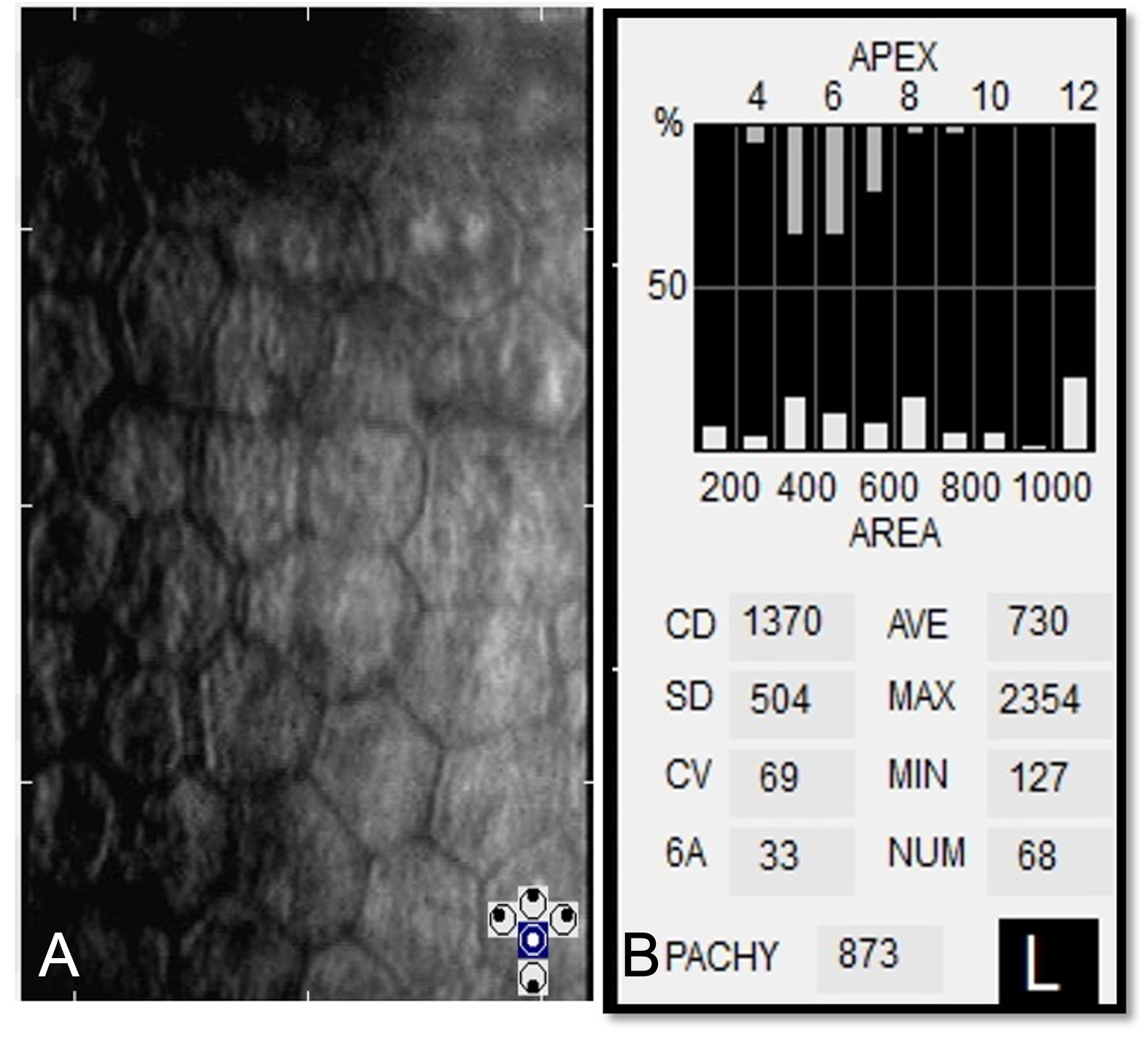
Fig 2A & 2B Specular microscopy shows polymegathism and pleomorphism. Note the increase in the mean cell area. 2B Shows increase in corneal thickness (pachymetry), decreased cell density, large CV, and decreased hexagonal cells
ASOCT:
Shows increased thickness with multiple epithelial bullae and irregularity at Descemet’s membrane and endothelium. No e/o any localised Descemet’s membrane detachment (DMD) or scarring.
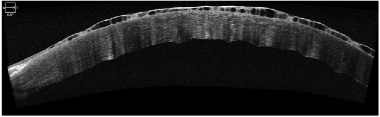
Fig. 3- ASOCT shows a horizontal section of the cornea with increased thickness of epithelial layer with the fluid collection and endothelial misalignment
Differential Diagnosis:
- Fuch’s endothelial dystrophy
- Herpetic stromal keratitis
- Aphakic bullous keratopathy
- Iridocorneal endothelial (ICE) dystrophy
- Congenital hereditary endothelial dystrophy
- Congenital stromal dystrophy
- Posterior Polymorphic Membrane Dystrophy
Clinical Course of the condition:
After taking into account detailed history, symptoms, BCVA, clinical picture, specular microscopy findings, and ASOCT, the patient was given a Bandage contact lens, local dehydrating agents (NaCl 5% eye drop) for a month. The patient was not relieved and underwent OS Descemet stripping endothelial keratoplasty (DSEK). 6 months post-surgery, the graft was clear (Fig 4), the BCVA was 6/12.
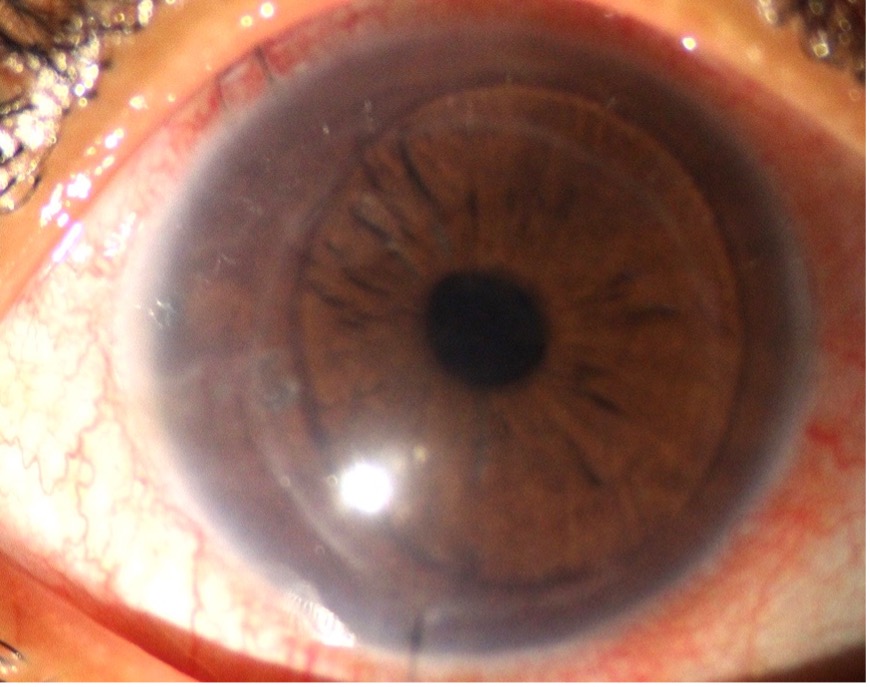
Fig. 3A- Shows clear DSEK lenticule at 6 months follow up
DISCUSSION:
Epidemiology
With current techniques of cataract surgery (using OVDs combined with posterior chamber IOL implant), reported incidence of pseudophakic bullous keratopathy ranges from 1 to 2%. The long-term reported incidence is about 15%. Previously certain intraocular lens designs, particularly angle-supported anterior chamber lenses, had up to 10% of corneal decompensation. Similarly, SICS has an incidence of 4.21% and Phacoemulsification 5.41% respectively. Although this may increase to 11 to 24% if the pre-op endothelial count is below 1000 cells/mm2
Etiology
- Pre-Operative
- Pre-existing low endothelial count
- Unhealthy endothelium secondary to ocular inflammation (previous trauma/surgery)
- Pseudoexfoliation endotheliopathy
- Intra-Operative-
- Endothelial damage due to eventful surgery such as DMD, endothelial touch, inexperienced surgeon, prolonged total surgical time, significant pupillary constriction, PC rent/vitreous disturbance, toxic intraocular medications etc.
- Corneal burn due to excessive phaco power
- Post-operative-
- Decentred/subluxated IOL, Type of IOL (Anterior chamber IOL>Iris fixated IOL> Posterior chamber IOL>scleral fixated IOL)
- Post-operative inflammation
- High intraocular pressure
- Systemic conditions (diabetes, chronic obstructive pulmonary disease, autoimmune diseases) cause pro-inflammatory states, alteration of local ocular immune status and poor wound healing along with imbalance in VEGF and anti VEGF factors.
Anatomy and pathophysiology
Normal corneal transparency is maintained by its anatomical and physiological factors. Anatomical factors include the typical arrangement of corneal epithelium, lamellae, and corneal avascularity. Physiological factors include relative corneal dehydration. Any disturbance in corneal anatomy or physiology will lead to a loss of corneal transparency. Multiple factors which affect corneal hydration are stromal swelling pressure by GAG of stroma, the barrier function of epithelium, and endothelium.
Central endothelial cell density decreases with age at an average rate of approximately 0.6% year, diminishing from a count of about 3400 cell/mm2 at age 15 years to about 2300 cells/mm2 at age 85 years. The corneal endothelial cells do not have regenerative capabilities and any cell loss is compensated by polymegathism and polymorphism.
Prolonged endothelial decompensation causes stromal edema as a result of the failure of the Na+-K+ ATPase pump followed by epithelial edema. Epithelial edema results in the formation of blebs and bullae, which can rupture during blinks or trivial trauma such as eye rubbing. Repeated episodes of epithelial loss cause constant stimulation of the limbal stem cells and gradual loss of function, subepithelial scarring ultimately leading to superficial corneal vascularization. Edema and persistent inflammation can lead to stromal vascularization, which in turn can lead to pannus formation and loss of transparency.
Clinical Features
Symptoms:
- Blurred vision more on awakening
- Glare and photophobia
- Foreign body sensation
- Severe pain and watering from ruptured epithelial bullae
- Loss of vision
Signs:
- Reduced contrast sensitivity
- Pigment dusting on endothelium
- Central microcystic epithelial edema
- Stromal edema and thickening
- Thickened Descemet’s membrane and folds
- Bullous keratopathy
- Subepithelial scarring and fibrosis
Ancillary Investigation
Specular microscopy:
Specular microscopy shows a magnified view of the corneal endothelium. Both qualitative and quantitative evaluation of the endothelium can be done, with cell density as a quantitative measure and CV and 6A showing pleomorphism and polymegathism for qualitative assessment. In PBK, both CV and 6A are deranged with decreased endothelial cell count. In advanced cases, specular microscopy is frequently not possible due to subepithelial and stromal scarring.
Serial pachymetry
Pachymetry is the measurement of the central corneal thickness, which is increased in PBK due to stromal edema. It helps in assessing the progression of disease as the cornea edema increases secondary to endothelial cell decompensation.
Anterior Segment Optical Coherence Tomography (ASOCT):
ASOCT is used for assessment of DM and to rule out any sub-epithelial or stromal scarring before planning for surgery. ASOCT can measure both the central and the peripheral corneal thickness and thus can be used as an indirect measure of corneal decompensation.
Management
Non-SurgicalManagement:
1. Topicals-
- Topical Hyperosmotic agents- 5% sodium chloride (eye drop/ointment) is used to reduce stromal edema. It creates a hypertonic tear film that draws water out of the cornea. Hypertonic saline helps in resolution of corneal edema in almost one-third of patients with early PBK. Not effective in advanced stage when scarring and vascularisation develops.
- Rho-kinase inhibitors-New and promising treatment for very early endothelial decompensation. Ripasudil(0.4%), Netarsudil(0.02%)4 times a day, inhibits apoptosis and promote endothelial cell proliferation. Molecular study revealsthat ROCK inhibitor increases cyclin D levels and suppresses phosphorylation of p27by activation of phosphatidylinositol 3-kinase signalling. As these two factors are regulators of the G1/S progression. Ki67-positive proliferating cells are also increased which suggests that ROCK inhibition promotes endothelial proliferation. ROCK inhibitor eye drops can promote the proliferation of the residual endothelium following corneal endothelial damage and increase the numbers of endothelial cells available for coverage, thereby reducing the risk of corneal decompensation.
2. Bandage contact lens
Combined with topical osmotic agents may be used for symptomatic relief as it reduces pain associated with bullae. However, a BCL needs to be changed at regular intervals. Prolonged use introduces the risk of infection.
3. IOL redialling/exchange
In very early cases with IOL haptic/Optic touching the endothelium just redialling the intraocular lens in its normal anatomical position or exchanging it with iris-claw/scleral fixated IOL can prevent long term corneal decompensation and reverse the corneal edema. Early intervention in such cases is beneficial.
4. Anterior stromal puncture
Puncturing the bullae with 24/26G needle under slit-lamp can relieve symptoms in patients not qualified for DSEK/OPK. About 150-200 punctures are given under topical anaesthesia. It is a simple cost effective treatment for symptomatic relief of patients. It reduces pain and watering in such patients.ASP promotes subepithelial scarring and hence reduces further chances of bullae formation.
5. Phototherapeutic keratectomy-
Superficial PTK (upto25 μm), intermediate (50–100 μm), or deep PTK (25% stromal thickness) using excimer laser after manual epithelial debridement has been tried for pseudophakic patients with poor visual outcome. Deep PTK involving at least 25% of stromal tissue is more effective in relieving symptoms as it treats the subepithelial nerve ending and reduces pain.
6. Conjunctival flap-
Rotation flaps with its different modifications for poor visual outcome patients. However, it distorts the normal anatomy of the conjunctiva inducing more scarring.
7. AMG+-BCL-
For symptomatic relief of poor visual prognosis cases on-lay technique of AMG after removing epithelium and securing it with 10-0 nylon can be used.
8. Collagen cross-linking-
Modified Dresden protocol has been recently tried with some limited success. Corneal epithelium debridement is done followed by instillation of riboflavin eye drops for 30 minutes. UVA radiations delivered for 10 minutes at 9 mW/cm2. It gives temporary symptomatic relief for patients.
Surgical Management
- Penetrating keratoplasty-
PK has been the definitive treatment for visual rehabilitation in patients with pseudophakic bullous keratopathy since a long time. It is still the preferred method of management in cases of severe scarring and vascularisation or where lamellar dissection is not possible. Full thickness penetrating keratoplasty has its own inherent disadvantages related to high risk of wound dehiscence, graft rejection, post-op astigmatism, sutures related complications, supra-choroidal hemorrhage and expulsive hemorrhage
- DSEK/DSAEK-
Descemet stripping endothelial keratoplasty/Descemet stripping automated endothelial keratoplasty is considered as surgery of choice for pseudophakic bullous keratopathy without any significant scarring or vascularisation. Here dysfunctional endothelium and Descemet membrane is replaced with healthy endothelium, DM and thin portion of posterior stroma. It is superior to penetrating keratoplasty in terms of early visual recovery, refractive stability, less postop astigmatism, no suture-related complications and minimum risk of expulsive hemorrhage as it is a closed globe surgery.
- DMEK-
Descemet Membrane endothelial keratoplasty, in this diseased endothelium and DM is replaced with donor DM-endothelium complex, without any donor stromal component. Fastest visual recovery can be achieved in this type of lamellar endothelial keratoplasty. DMEK lenticule is inserted using an injector. However, disadvantage is that corneal tissues younger than 45 years are difficult to use as DM is strongly adherent to overlying stroma in young patients. Older tissues are easier to use but they have a low endothelial count and thus have theoretically less longevity.
- PDEK-
Pre-Descemet's EK (PDEK) is a newer variant of EK. It Involves separation of Pre-Descemet layer along with DM-endothelium as donor lenticule by formation of a Type 1 big bubble. So younger tissues can also be used for transplantation in contrast to DMEK.
- Cultured corneal endothelial sheet transplantationhas been tried with limited success.
Summary
Etiology:
|
Signs:
|
Symptoms:
|
Investigations:
|
Treatment:Non-Surgical: 1. Topicals- (A)Topical Hyperosmotic agents- Nacl 5% eye drop/ointment (B)ROCK inhibitors-Ripasudil(0.4%), Netarsudil(0.02%) 4 times a day 2. BCL 3. Anterior stromal puncture+BCL 3. Amniotic membrane grafting 4. Conjunctival flaps Surgical Management 1.Penetrating keratoplasty 2. Descemet Membrane Endothelial keratoplasty (DMEK) 3. Descemet Stripping Endothelial Keratoplasty (DSEK) 4. Pre-Descemets stripping endothelial keratoplasty(PDEK) |
Differentials:
|
References
- Basak SK, Basak S. Complications and management in Descemet's stripping endothelial keratoplasty: Analysis of consecutive 430 cases. Indian J Ophthalmol 2014;62:209-18.
- Naoki Okumura et al, Application of Rho Kinase Inhibitors for the Treatment of Corneal Endothelial Diseases, Hindawi Journal of Ophthalmology Volume 2017, Article ID 2646904, 8 pages.
- Agarwal A, Dua HS, Narang P, Kumar DA, Agarwal A, Jacob S, Agarwal A, Gupta A. Pre-Descemet's endothelial keratoplasty (PDEK). Br J Ophthalmol. 2014 Sep;98(9):1181-5.
- Agrawal V, Vagh MM, Sangwan V, Rao GN. Penetrating keratoplasty for pseudophakic bullous keratopathy.Indian J Ophthalmol 1994;42:75-80
- Pricopie S, Istrate S, Voinea L, Leasu C, Paun V, Radu C. Pseudophakic bullous keratopathy.Rom J Ophthalmol. 2017;61(2):90-94. doi:10.22336/rjo.2017.17
- A K Khurana, Anatomy and physiology, 3rd ed. Pg 22-37
- Narayana R, Gaster R, and Kenney M. Pseudophakic Corneal Edema: A Review of Mechanisms and Treatments. Cornea. 2006; 25(9): 993-1004.
- Ganekal S, Nagarajappa A. Comparison of morphological and functional endothelial cell changes after cataract surgery: Phacoemulsification versus manual small-incision cataract surgery. Middle East Afr J Ophthalmol 2014;21:56-60.
- Singhal D, Sahay P, Goel S, Asif MI, Maharana PK, Sharma N. Descemet membrane detachment. SurvOphthalmol. 2020 May-Jun;65(3):279-293. doi: 10.1016/j.survophthal.2019.12.006. Epub 2020 Jan 8. PMID: 31923476.
- Narang, Priya; Agarwal, Amar1,Pre-Descemet's endothelial keratoplasty, Indian Journal of Ophthalmology: June 2017 - Volume 65 - Issue 6 - p 443-451 doi: 10.4103/ijo.IJO_324_17.

