In early days, since the whole lenses were readily available from intracapsular cataract surgery, so the early systems of cataract classification1,2,3 were developed for use almost exclusively with isolated lenses. These systems were largely qualitative, and they emphasized nuclear color as the index of the severity of cataract formation; lighting systems that improved visualization of cortical and subcapsular morphologic detail had not been developed.
Before 1976, lens and cataract research emphasized studies of animal lenses, and researchers erroneously believed these animal lenses to be adequate models of the human lens. In 1976, the CCRG was established to increase understanding of the mechanisms of human cataract formation. One of the CCRG's goals was to establish a lens classification system to help lens researchers study the biochemical and biophysical properties of the cataractous lens and compare them with those of normal lenses. Such a system would facilitate biochemical, biophysical, anatomic, and pathologic correlations.
The first comprehensive classification system was introduced in 19784 and adopted by the American CCRG in 1980.5 The system divides the lens into two distinct zones: the cortex and the nucleus. The cortex is further subdivided into six anatomic zones: subcapsular anterior (SCA), subcapsular posterior (SCP), anterior cortical (CXA), equatorial cortical (CXE), posterior cortical (CXP), and supranuclear (SN), a zone found between the cortical and the nuclear zones.6 The nuclear (N) region is not subdivided. Subscripts are added to the classification terms to indicate the extent or intensity of involvement in that particular region of the lens. For CXA, CXE, CXP, and SN, each zone is subdivided into four quadrants, and the total extent of opacification, even if scattered throughout the zone, is expressed in terms of the number of quadrants that are totally opacified; these are denoted by subscripts 1 to 4. For SCA and SCP, the extent of opacification is estimated by comparing the opaque zone with the area within each of a series of concentric circles,7representing opacification of 3, 4, 25, 44, 69, and 100% of the area of the equatorial circle of the lens. The percentage of the total area involved is denoted as a subscript. Although the CCRG classification system was designed primarily for use on extracted lenses; it proved useful to some ophthalmologists for in vivo classification.
In Vivo Cataract Classification Systems
The constant need for a valid and reproducible system for in vivo cataract clasfication system was felt by many ophthalmologists and several publications emphasized the need for the same.8,9,10 One of the earliest challenges was to generate good photographs of cataracts that might serve as reference standards. Once standards were available, it was necessary to assess the reproducibility of newly developed cataract classification systems.
Investigations turned to photographic techniques that yielded nearly artifact-free hard copy of retroillumination and slit images of cataracts. With these images, interobserver and intraobserver consistency could be measured easily. The photographic images that proved most useful to lens classifiers were Topcon SL-45 Scheimpflug black-and-white slit images, Neitz CTR retroillumination images, and Zeiss color slit images of the lens. With the advent of digital imaging technology, the Scheimpflug and retroillumination optics were incorporated into two digital camera systems: the Nidek EAS-100011 and the Marcher Industries Case-2000.12. Both these instruments characterize the severity of nuclear cataract in terms of nuclear density and cortical and posterior subcapsular cataract in terms of the percentage of the area that is opaque (the image is binarized into a clear zone and an opaque zone on the basis of a threshold set either by the instrument or by the examiner). These digital instruments, aside from being elegantly designed and produced, are very examiner- and subject-friendly. They also allow one to avoid using film, with all the needs for standardization of development, storage, and analysis, and use electronic image storage, analysis, and data manipulation.
Several similar in vivo cataract classification systems have been developed.13 14, 15, 16, 17, 18, 19, 20, 21, Each system uses standard photographs that represent boundaries between different degrees of opacity. By comparing the patient's lens under defined lighting conditions with the standard photographs, a trained observer can define the type and extent of opacity in each lens zone. Systems differ not only in the number of photographs in each class of standards but also in the features that are emphasized as defining age-related cataract. For example, the LOCS II system employs one nuclear color standard and five cortical, four posterior subcapsular, and four nuclear opalescence standards.12 The LOCS III system employs six nuclear standards for color and opalescence grading and five standards for cortical and posterior subcapsular grading.7 The Wilmer system uses four nuclear standards but does not grade color.14,19 The Oxford system isolates and grades features that are included in the general categorycorticalin LOCS..22 The Oxford system grades nuclear color change but uses a set of Munsell color chips rather than images of nuclear cataract. The Japanese system developed by Sasaki and colleagues offers a new set of standards for grading nuclear cataracts.20, 21.
Also developed were simple, comprehensive, and standardized classification and grading systems for use at the slit lamp. A classifier trained in the LOCS system, e.g., could classify patients' lenses at the slit lamp or in standard photographs using essentially identical criteria.
LENS OPACITIES CLASSIFICATION SYSTEMS I, II, AND III
The most widely used classification system in the United States is the Lens Opacities Classification Systems I to III, which has been used in several national and international collaborative projects. LOCS uses a reference set of standard photographs that defines theextentof opacification in the cortical (C) and posterior subcapsular (P) zones, and theintensityof nuclear opalescence (NO) in the nuclear zone. Also in LOCS, nuclear color (NC) is graded separately from opalescence.
LOCS I was originally designed for use in a case-control study of risk factors for age-related cataract sponsored by the National Eye Institute.14 Recruited for the study were patients with cataract and control subjects without cataract. At the time of the study visit, the ophthalmologist had to classify the type and severity of all lens opacities. These classification data and other epidemiologic information were used to identify risk factors for specific types and extents of cataract.23 In LOCS I, an ordinal scaling ranging from zero (no opacification) to 2 (definite opacification) for each class defines the severity of opacification or intensity of brunescence. A grade of zero implies the absence of lens opacities; a grade of 1 implies the presence of early opacification; and a grade of 2 implies definite cataract. Because of the high frequency of minor, age-related cortical lens changes, the system subdivides early cortical opacification into 1a and 1b classes. The category 1a includes minor, clinically insignificant opacification; the category 1b includes early cortical cataract.
The boundaries between the gradings are defined by a set of standard photographs, consisting of one slit-lamp color photograph used to grade nuclear opalescence and nuclear color and three black-and-white retroillumination photographs used for posterior subcapsular and cortical classifications. The standard photographs are reproduced on an 81/2- by 11-inch transparency and placed on a light box located at eye level behind the patient's right shoulder when the patient is seated at the slit lamp. The classifier can easily refer to the standards during the examination, which is done with the patient's pupils maximally dilated.
LOCS II is an expanded version of LOCS I, designed to further differentiate among the various degrees of cortical, subcapsular, and nuclear opacification.12 It was also designed for use in longitudinal studies. Colored standards are used, and the number of reference standards is increased. LOCS II uses four nuclear standards for grading nuclear opalescence, five cortical standards, and four subcapsular standards. The standards demarcate boundaries between grades.
Nuclear color (NC) is graded by comparing the color of the posterior cortical–posterior subcapsular reflex to the nuclear I (NI) standard, which is the same standard as in LOCS I. The biomicroscopist uses the low-magnification view of the slit lamp with the slit beam oriented approximately 45 degrees to the patient's visual axis, and the slit height and brightness are set to equal those in the standard photograph. If the color is less yellow than the standard, the nuclear color is graded zero; if it is similar, it is graded 1; and if it is darker yellow, it is graded 2.
In grading nuclear opalescence (NO), the classifier estimates the average opalescence of the nucleus (the overall density of the entire lens enclosed within the zones of supranuclear scattering) and determines whether it is equal to or less than a specific nuclear standard. For example, if the average NO is more than the N0 but less than or equal to the NI standard, the grade is NI.
When grading a cortical (C) or posterior subcapsular (P) opacity, the grader estimates whether the aggregate area of such an opacity is less than or equal to the aggregate area of the opacity in a particular standard. The classifier envisions an aggregate opacity by clumping all contiguous and noncontiguous opacities into one zone. The size of the opaque zone relative to the size of the opaque zone in the standards determines which class is chosen to grade the cataract. In the LOCS classification scheme, the cortical and posterior subcapsular zones are graded individually as C and P. The C zone includes the subcapsular anterior, cortical anterior, cortical equatorial, cortical posterior, and supranuclear zones of the original American CCRG in vitro classification scheme; the P zone includes the subcapsular posterior zone. The classification data are recorded on a form that contains guidelines for classifying each type of opacification
Wilmer System
The Wilmer system uses four nuclear standard photographs for grading nuclear opacity.14, 19 The gradings are based on three criteria: visual acuity, density, and extent of the opacity. presents the nuclear standards 1 to 4, and illustrates the nuclear opacity grading definitions.14 The nucleus is examined with a thin slit beam that passes through the center of the lens, and the clarity of the optical section of the nucleus is compared with the standard photograph and graded accordingly. The cortical and posterior subcapsular opacities are examined using retroillumination. Only opacities that could be seen in retroillumination are graded by estimating the proportion of the total circumference of the lens occupied by the combined cortical opacities as if they are adjacent, gives the cortical opacity grading definitions. 14 The posterior subcapsular opacities are assessed by determining the overall vertical and horizontal dimensions measured using the calibration of the slit-beam height. These dimensions are then multiplied to give an approximation of area. Intraobserver and interobserver agreements are good, with the exception of the interobserver agreements for posterior subcapsular cataracts. 14 The Wilmer system has been used in studies by Adamsons and colleagues and Taylor and associates.15,16,17,18
Oxford System
Oxford system is a very comprehensive system of classifying and grading cataracts. It includes quantitation on a scale of zero to 5 of such features as anterior clear zone thickness, anterior subcapsular opacity, posterior subcapsular opacity, cortical spoke opacity, waterclefts, vacuoles, retrodots, focal dots, nuclear brunescence, and white nuclear scatter.22 The system gives theanterior clear zone thicknessreference standards, thediameter scalereference standards for grading anterior and posterior subcapsular opacities, thepie segmentreference standards for grading spoke opacities and waterclefts, thevacuolereference standards, theretrodotreference standards, thefocal dotreference standards. 22 Nuclear brunescence is graded by comparing the posterior region of the nucleus just anterior to the posterior nuclear shell with a set of Munsell color chips. The grade is selected according to the closest available match between the nucleus and the color chips 22 White nuclear scatter is assessed as the amount of white light being scattered back to the observer by the lens nucleus and is compared with Munsell neutral-density gray-scale samples 22
Japanese Cooperative Cataract Epidemiology Study Group System
The Japanese Cooperative Cataract Epidemiology Study Group system also uses a set of standard photographs in grading cortical, nuclear, and subcapsular opacities.24 These cataracts are graded as early (I), moderate (II), or advanced (III). and give the standard photographs of cortical and nuclear cataract, respectively. 24 An opacified area corresponding to the normal pupil size is graded I; an area larger than a normal pupil but smaller than a moderately dilated pupil as II; and one larger than a moderately dilated pupil as grade III. Subcapsular opacities are graded in reference to the pupil size. Nuclear coloration is classified into four gradings: I, pale yellow; II, yellow; III, brownish-yellow; IV, brown (including reddish-brown and black-brown The Japanese Cooperative Cataract Epidemiology Study Group system also uses a set of standard photographs in grading cortical, nuclear, and subcapsular opacities
World Health Organization's Simplified Cataract Grading System
There had been so many classification systems with their own merits and demerits so in an attempt to unify the methods of grading age-related cataract and produce a simplified version of present grading systems, the WHO in 1996 convened a meeting of most of the clinicians and epidemiologists with cataract classification expertise. The charge to this group was to design, implement, and validate a new system of classifying age-related cataract using the strengths of the existing systems of cataract classification. This goal has been largely achieved and at present this system is undergoing field testing in Australia. When testing is completed, test results and details of the system will be published.
Recent Attempts at Classification Of Cataracts
A study was published in British Journal of Ophthalmology in 2008, which set to find out the correlation of lens density measured using the Pentacam Scheimpflug system with the Lens Opacities Classification System III grading score. It incorporated 180 eyes of 138 patients whose visual acuity was testing using the ETDRS chart and the LOCS III grading and Pentacam evaluation was done. It was found out that there was a linear increasing relationship between lens density value andLOCSIII grading score in nuclear cataract patients. Lens density value had a stronger significant correlation withLOCSIII NO score than that with NC score. The correlation between the nuclear lens density value and log MAR visual acuity was stronger than that between NO score and log MAR visual acuity or between NC score and log MAR visual acuity 25
Another study published in Ophthalmology in 200926 also proved the correlation of LOCS III with pentacam Schiempflug imaging system. 110 patients were enrolled in the study and it was found out that certain areas of nucleus are more representative of the lens density on schiempflug imaging (region of interest), and that these areas correlated with the LOCS III, whereas the Average Lens Density measurement on Pentacam did not correlate with the LOCS III standards. (Figures 1-3)
Figure 1 : Oculus Pentacam
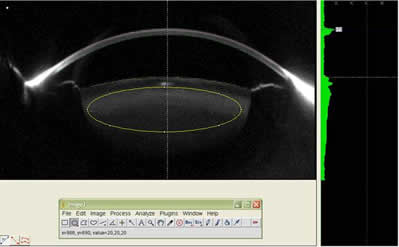
Figure 2: The figure shows a Region Of Interest(ROI) being measured with the help of ImageJ software
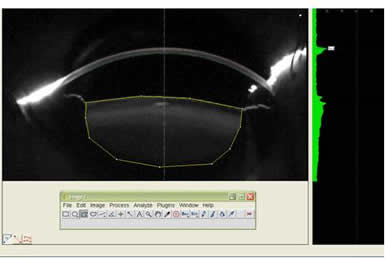
Figure 3: The figure shows Average Lens Density being measured with the help of ImageJ software
References
1. Pirie A: Color and solubility of the proteins of human cataracts. Invest Ophthalmol1968; 7:634
2. Truscott RJW, Augusteyn RC: Changes in human lens proteins during nuclear cataract formation. Exp Eye Res 1977; 24:159
3. Marcantonio JM, Duncan G, Davies PD, et al: Classification of human senile cataracts by nuclear colour and sodium content. Exp Eye Res 1980; 31:227
4.Chylack LT Jr: Classification of human cataracts. Arch Ophthalmol , 1978
; 96:888
5.Chylack LT Jr, Lee MR, Tung WH, Cheng HM: Classification of human senile cataractous change by the American Cooperative Cataract Research Group (CCRG) method. I: Instrumentation and technique. Invest Ophthalmol Vis Sci 1983; 24:424
6. Chylack LT Jr, Lee MR, Tung WH, Cheng HM: Classification of human senile cataractous change by the Americal Cooperative Cataract Research Group (CCRG) method. I: Instrumentation and technique. Invest Ophthalmol Vis Sci , 1983; 24:424
7. Chylack LT Jr, Wolfe JK, Singer DM, et al: The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993; 111:831–836
8.Duncan G: On classifying human cataractous lens. In Duncan G (ed): Mechanisms of Cataract Formation in the Human Lens. Orlando, FL, Academic, 1981.
9. Leske MC, Sperduto R: The epidemiology of senile cataracts: A review. Am J Epidemiol, 1983; 118:152,
10. West SK, Taylor HR: The detection and grading of cataract: An epidemiologic approach. Surv Ophthalmol , 1986; 31:175
11. Baez KA, Orengo S, Gandham S, Spaeth GL: Intraobserver and interobserver reproducibility of the Nidek EAS-1000 Anterior Eye Segment Analysis System. Ophthalmic Surg , 1992; 23:426–428
12.Chylack LT Jr, Leske MC, McCarthy D, et al: Lens Opacities Classification System II (LOCS II). Arch Ophthalmol, 1989; 107:991.
13 Chylack LT Jr, Leske MC, Sperduto R, et al: Lens Opacities Classification System. Arch Ophthalmol , 1988; 106:330
14. Taylor HR, West SK: A simple system for the clinical grading of lens opacities. Lens Res , 1988; 5:175
15. Adamsons I, Taylor KI, Enger C, Taylor HR: A new method for documenting lens opacities. Am J Ophthalmol, 1991 111:65–70
16. Adamsons I, Munoz B, Enger C, Taylor HR: Prevalence of lens opacities in surgical and general populations. Arch Ophthalmol , 1991; 109:993–997
17. Lee JA and Taylor HR: Evaluation of photographic methods for documentation of lens opacities. Invest Ophthalmol Vis Sci, 1990 31:1191–1193
18. Taylor HR, Munoz B: The incidence and progression of lens opacities. Aust N Z J Ophthalmol, 1991; 19:353–356
19. West SK, Rosenthal F, Newland HS, et al: Use of photographic techniques to grade nuclear cataracts. Invest Ophthalmol Vis Sci, 1988 29:73
20. Sasaki K, Shibata T, Obazawa H, et al: A cataract classification and grading system. Nippon Ganka Gakkai Zasshi 1989; 93:796–800
21. Sasaki K, Sakamoto Y, Fujisawa K, et al: A new grading system for nuclear cataracts—An alternative to the Japanese Cooperative Cataract Epidemiology Study Group's grading system. Dev Ophthalmol , 1997;
27:42–49
22. Sparrow JM, Bron AJ, Brown NAP, et al: The Oxford clinical cataract classification and grading system. Int Ophthalmol, 1986; 9:207–225
23. Leske MC, Chylack LT Jr, Wu SY, et al: The lens opacities case-control study: Risk factors for cataract. Arch Ophthalmol , 1991; 109:244
24. Sasaki K, Shibata T, Obazawa H, et al: Classification system for cataracts: Application by the Japanese Cooperative Cataract Epidemiology Study Group. Ophthalmic Res , 1990; 22(Suppl 1):46.
25. X Pei, Y Bao, Y Chen and X Li : Correlation of lens density measured using thePentacamScheimpflug system with the Lens Opacities Classification System III grading score and visual acuity in age-related nuclear cataract. British Journal of Ophthalmology 2008;92:1471-1475
26. Dilraj S. Grewal , Gagandeep S. Brar, Satinder Pal Singh Grewal :Correlation of Nuclear Cataract Lens Density Using Scheimpflug Images with Lens Opacities Classification System III and Visual Function. Ophthalmology 2009;116:1436-1443
