Epiretinal membranes(ERM) are acquired formation of semi-transparent cellular sheets on the macular surface, which are formed due to varied etiologies. These membranes have contractile properties, often leading to mechanical distortion of macula.
The proliferation of ERM on the inner retinal surface along the internal limiting membrane was first described by Iwanoff in 18651.In 1971, Roth and Foos reported that in a series of autopsied eyes, ERM were present in 2 percent of patients aged 50 yr and in more than 20 percent of patients by age 75 yr2. Pearlstone reported an incidence of 6.4 percent in 1000 consecutive routine eye examinations in patients older than 50 yr, with 20 percent being bilateral3,4.Other authors report bilateral involvement in 10 to 20% of cases5,6,7.The Los Angeles Latino Eye Study (LALES) found that of the participants with ERM, 19.9% had bilateral ERMs. The prevalence of ERM increased from 10.1% in persons 40 to 49 years of age to 35.7% in those aged 70 to 79 years and was 22.5% in persons aged 80 years or more. The prevalence was similar in men and women. ERMs were present in 71% of eyes with macular holes and were more common in individuals who had undergone cataract surgery (39.9%), those with proliferative diabetic retinopathy (25.7%), and those with any retinal disease (27.5%).8
The wrinkling of the retinal surface caused by ERMs in the macular region has been termed surface wrinkling retinopathy,2macular pucker,9–10cellophane maculopathy,11, 12 wrinkling of the internal limiting membrane,13preretinal macular fibrosis,13,14 epiretinal macular membrane, primary retinal folds,15internoretinal fibrosis,16 idiopathic preretinal macular gliosis,6,17,18undetected central retinal vein occlusion,19 and silkscreen retinopathy. The term macular pucker has most frequently been applied to the condition when retinal surface wrinkling occurs following reattachment of a rhegmatogenous retinal detachment. This condition complicates 4 to 8 percent of otherwise successful primary retinal reattachments.20,21
ETIOLOGICAL CLASSIFICATION:
Primary ERMsare idiopathic in nature and are seen in patients over 50 years of age. Anomalous posterior vitreous detachment (PVD), has been described to be important in the pathogenesis of idiopathic epiretinal membrane, reported having been present in 75% of cases.2-6,11,22,23It constitutes of (1) vitreous liquefaction with an accumulation of fluids in the premacular portion of the vitreous cavity; (2) varying degrees of persistent adhesion between the posterior cortical vitreous and the internal limiting membrane (ILM); (3) splitting, or “vitreoschisis,” of the posterior cortical vitreous at the time of PVD; and (4) residual islands of cortical vitreous attached to the ILM following separation of the majority of the gel from the surface of the retina24.
Secondary ERMshave been found in association with retinal vascular deseases, retinal breaks and detachments25, ocular trauma, intraocular inflammation (Pars planitis/uveitis/Vitritis, CME)26, uveitis, and following retinal cryopexy, laser photocoagulation27, and intraocular surgery. Both types of epiretinal membranes have a similar clinical appearance and may be differentiated on the basis of history or other associated features. Clarkson and coworkers, in a review of 1612 postmortem eyes, noted epiretinal membranes in 1.7 percent of eyes that had not undergone previous ocular surgery3. In one study it was found that 46.2% of patients with epiretinal membranes were idiopathic while 53.8% had epiretinal membranes following previous vitreoretinal surgery. Main reasons for vitreoretinal surgery in this group was – 60% complications of diabetic retinopathy, 2.8% endophthalmitis after cataract surgery, 5.7% uveitis, 14.2% retinal detachment, 8.6% trauma and 8.6% vitreous hemorrhage because of retinal vein occlusion28. The prevalence of ERMS formation has been shown to increase by 71.4% during the first 6 months after uneventful ECCE with PC IOL implantation. Authors concluded that ERMs were probably induced by the uneventful surgery29. Following pars plana vitrectomy (PPV) for repair of primary rhegmatogenous retinal detachment 12.8% eyes were noted to have a postoperative epiretinal membrane, clinically30. ERM is also rarely reported with retinal tumors (neurofibromatosis31, vasoproliferative tumors32, macro aneurysms), drugs (NSAID’s. Tamoxifen, PFCL, S.O.).
Clinical features
Patients with epiretinal membranes peripheral to the macula are generally asymptomatic. When the membranes involve the macula or are perimacular, however, the type and degree of symptoms experienced will depend on the membrane thickness, the degree of retinal distortion caused by the overlying membrane, the presence or lack of significant traction that can cause a micro detachment of the posterior pole, and the presence or lack of edema in the macular and perimacular regions.
Thin epiretinal membranes usually cause few symptoms. The condition appears to be relatively stable or slowly progressive with only a small number of patients, approximately 5 percent, having a vision of 20/200 or worse.5,33,34 In more advanced cases, there is a reduction in vision, micropsia, metamorphopsia, Amsler grid distortion, and occasionally monocular diplopia. Spontaneous separation of an epiretinal macular membrane, although uncommon(1%), can occur; when this happens, there is a general decrease in symptoms and a concomitant improvement in visual acuity.12, 15, 35–38
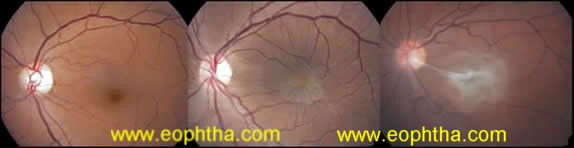
Gass in 1987 classified ERM in three grades(Fig 1).
Grade 0: cellophane maculopathy,characterized by translucent membranes with no retinal distortion or obscuration of underlying vessels. There may be a irregular reflex, mild sheen or glint, without a distinct edge,
Grade 1: crinkled cellophane maculopathy, causing irregular wrinkling of inner retina but underlying vessels are still visible. They cause increased vascular tortuosity, with perimacular vessels seen to be pulled toward an epicenter and
Grade 2: macular puckerwith opaque membranes obscuring underlying vessels, prominent retinal distortion and arcuate vessels are closer togather, often leading to foveal ectopia , heterotopia or shallow tabletop retinal detachment.
Other findings that may be present include small intraretinal hemorrhages, cystic changes in the macula, cotton wool spots39 and macular edema. Pseudoholes or macular cysts have been noted in up to 8 percent of idiopathic cases.6,40The thicker and occasionally pigmented membranes are often seen following retinal detachment surgery, severe inflammatory conditions, and trauma.
Figure 1a,b,c: Cellophane, Crinkled cellophane maculopathy and Macular pucker
Pathology
The source of the cells producing these membranes has been controversial. Early reports suggested endothelial cells1, extension of muller cells process41, fibroblasts in the vascular connective tissue42, pigmented or nonpigmented cells of the pars ciliaris, inflammatory cells within the vitreous, or retinal glial cells43, etc as source of ERMs. Later, vitrectomy specimens have shown that epiretinal membranes comprise of glial cells, retinal pigment epithelial cells, macrophages, fibrocytes, and collagen cells. These cells are found in varying proportions in accordance with the etiology of the membrane. Foos suggested that the glial cells found in the thin idiopathic membranes were derived from the glial cells of the superficial retina and had migrated through breaks in the internal limiting lamina to proliferate on retinal surface44. Smiddy and associates suggest that the retinal pigment epithelial cells gain access to the retinal surface by various methods, including migration through occult breaks, inactivation of developmental rests of retinal pigment epithelial cells already on the surface of the retina, transformation from other cell types, or via transretinal migration.45Stern and coworkers46 suggested that the contractile forces of the membranes were related to their constituent cell types and were not dependent on intercellular collagen as suggested by previous investigators. The general consensus is that membranes associated with retinal breaks, previous retinal detachments, or cryopexy are composed mainly of dispersed RPE cells, while cells of glial origin predominate in the idiopathic ERM.45, 47
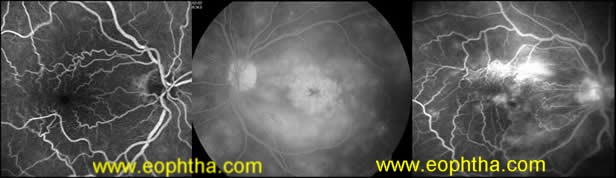
Fluorescein Angiography(fig 2)in cases of ERMs does not contribute anything significant in its diagnosis since the clinical picture is often specific enough. FA can help in assessing the degree of vascular tortuosity and tethering, extent of wrinkling, vascular leakage and macular edema. Vascular leakage when present is irregular, asymmetric and corresponds to the area of the ERM. It can also be done to differentiate pseudohole (absence of window defect) from true macular hole or rule out other lesions that may mimic ERM such as SRNVM, etc.
Figure 2: Angiographic features of ERM
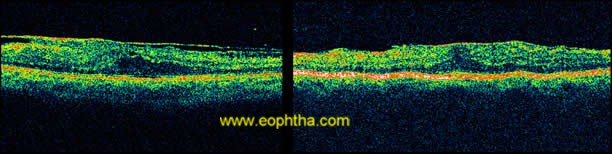
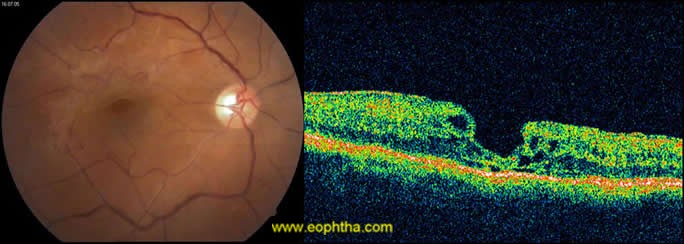
OCTdemonstrate ERM as thin hypereflective band over the surface of retina(fig 3).Majority (70%) are globally adherent to the retina while rest are focally adherent. OCT image of secondary ERM typically demonstrates diffuse thickening with loss of foveal pit. In idiopathic ERM mean central macular thickness correlates with visual acuity48. OCT helps in quantitative measurement of retinal thickness, membrane thickness, presence of CME, extent and adhesion points of membranes to the retinal surface, differentiates pseudohole from true macular holes(fig 4), lamellar holes, and macular cysts. It also provides beneficial information in monitoring surgical removal of ERM(fig 5)and decrease of intraretinal oedema after the surgery.49Tsuchihashi Takashi50has classified ERM on findings based on OCT as
1.Total adhesion with patent foveal pit,
2.Total adhesion with absent foveal pit,
3.Pseudohole,
4.Partial adhesion
Figure 3: OCT showing ERM as hyper-reflective band
Figure 4: CF and OCT of Pseudohole.
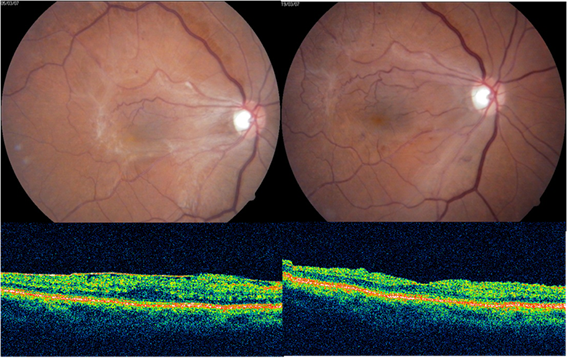
Figure 5: Pre and post operative CF and OCT of ERM
Treatment:
The majority of patients with epiretinal macular membranes have symptoms that are mild and either non progressive or slowly progressive; therefore, treatment is rarely indicated. The reduced visual acuity in patients with ERM may be due to dense membrane overlying macula, inner retinal layer distortion, full thickness retinal folds, trampoline like sensory foveal elevation, foveal ectopia, foveal retinoschisis, macular oedema, and traction induced ischemia and stasis of axoplasmic flow and they may need intervention.
Treatment for all patients with macular pucker is usually not necessary, since many may remain asymptomatic. There is no effective treatment available for mild forms of epiretinal macular membranes. Nsaids can be given to decrease the inflammation.
In 1978, Machemer51first reported on the surgical management of advanced cases of epiretinal macular membranes using pars plana vitrectomy. Subsequent authors reported on larger series and attempted to identify preoperative factors that appeared to influence the postoperative visual prognosis.49,52,53,54,55,56,57,58Although improvement in acuity is achieved in most cases, it may be less than expected, and it is rare to completely eliminate metamorphopsia. This appears to be especially true in eyes with relatively good preoperative vision.59,60Therefore, when vision is better than 20/70, surgery should be approached with caution.
Vitreous surgeons use conventional pars plana vitreous surgical techniques with either a three-port system or a two-port system utilizing a fiberoptic light pipe fitted with an infusion sleeve or 23 gauze sutureless vitrectomy. The surgical technique for removing epiretinal macular membranes includes first performing a limited posterior core vitrectomy. There is no need to remove the anterior vitreous, which may cause inadvertent lens damage in phakic patients and may increase the incidence of peripheral retinal tears or detachments, or both, in aphakic or pseudophakic patients. Their are various techniques reported for ERM peeling likeoutside – in technique (if edge is seen) , inside - out technique (no edge ), Rag-snag technique , using soft tip extrusion cannula , Pinch technique, Delamination /truncation with scisssors where they are tightly adherent or viscoelastic /saline dissection.
ERM removal basically involves identifying the edge of the membrane or creating one with the use of a blunt-tipped pick or a bent needle, MVR, diamond dusted Tano’s scrapper etc. Once the edge of the membrane is seen, it may be gently lifted off the retinal surface with the use of a pick or fine forceps. The membrane should be lifted in a tangential rather than an anteroposterior fashion so as not to pull on the underlying retina and create tears. Multiple graspings of the membrane should take place as close to the membrane- retina interface as possible to decrease shredding and allow better control of the dissection especially near the fovea. Dissection should start over the thickest part of the membrane or atleast in the most refractile portion of the membrane as they are tenaciously adherent at these palces and not over a major blood vessel especially in long standing and tightly adherent membranes. Once removed, underlying retina may have a whitish sheen and represents axoplasmic flow stasis and disappears within 48-73 hrs. Starting membrane dissection in areas of axoplasmic stasis is avoided because one may misinterprete it as additional layer of membrane. If one feels, additional force may tear the macula, the membrane should be re-engaged at other site and a different directional vector force used to strip the membrane. Rarely where the membranes cannot be removed, horizontal scissors are used to truncate the membrane close to the retina
ERM removal can also be done using fluidic internal limiting membrane separation (FILMS) cannula. The FILMS cannula is inserted under the ILM in the peripheral macula and Viscoelastic fluid is slowly injected using foot pedal control of a viscous fluid injector establishing a cleavage plane between the ILM and the remaining neurosensory retina. A cyst develops but at a rate controlled by the surgeon. With this technique no petechial hemorrhages are seen probably reflecting the fact that there is no mechanical pulling on the retina, but rather gentle tamponade of the retina, as the ILM/ERM complex is elevated. The separated tissue is then easily removed with forceps.
Whatever the technique used , the area within vascular arcades should be free of traction from ERM at the end of surgery. A thorough search for peripheral breaks shoud be done and retinopexy shoul d be done, if present.
Although technique and instrumentation have improved over the years, almost eliminating complications such as iatrogenic retinal tears and detachments, vitreous hemorrhage the problems of membrane recurrence61 and accelerated nuclear sclerosis remain unsolved.60,62,63,64The membrane recurs in less than 5 percent of idiopathic cases. In eyes with membranes from known causes, recurrence is much higher and can approach 100 percent in eyes of young patients in whom epimacular membranes develop following trauma or inflammatory disease61,62
Various vital stains like trypan blue which stains ERM or ICG which stains ILM and thereby helps in identifying ERM by negative staining can be utilized for complete removal or ERM. Triamcinolone acetonide also stains ERM and posterior hyaloid without the side effects of vital dyes and is used widely for this purpose.
Recent development in the concerned feild involves enzymatic induction of PVD with help of plasmin and chondroitinase, Erbium-YAG Laser photoablation(2.94µ). Macular epiretinal membranes have recently been managed with nonvitrectomizing vitreous surgery65, with the help of a proprietary microhooked needle with the benefit of non progression of cataract.
References:
1. Iwanoff A: Beiträ{umlaut-a}ge zur normalen und pathologischen Anatomie des Auges. Graefes Arch Clin Exp Ophthalmol 11:135–170, 1865.2. Roth AM, Foos RY: Surface wrinkling retinopathy in eyes enucleated at autopsy, Trans Am Acad Ophthalmol Otolaryngol 75:1047–1059, 1971.
3. Clarkson JG, Green WR: A histopathologic review of 168 cases of preretinal membrane. Am J Ophthalmol 84:1–17, 1977.
4. Pearlstone AD: The incidence of idiopathic preretinal macular gliosis. Ann Ophthalmol 17:378–380, 1985.
5. Scudder MJ, Eifrig DE: Spontaneous surface wrinkling retinopathy. Ann Ophthalmol 7:333–336, 339–341, 1975.
6. Sidd RJ, Fine SL, Owens SL, and Patz A: Idiopathic preretinal gliosis. Am J Ophthalmol 94:44-48, 1982.
7. Spitznas M, Leuenbeer R: Die primare epiretinale gliose. Klin Monatsbl Augenheilkd 171:410–420, 1977.
8. Samantha Fraser-Bell,Mei Ying-Lai Ronald Klein, and Rohit Varma,Prevalence and Associations of Epiretinal Membranes in Latinos: The Los Angeles Latino Eye Study Investigative Ophthalmology and Visual Science. 2004;45:1732-1736.
9. Tanenbaum HL, Schepens CL, Elzeneiny I, Freeman HM: Macular pucker following retinal detachment surgery. Arch Ophthalmol 83:286–293, 1970.
10. Tanenbaum HL, Schepens CL, Elzeneiny I, Freeman HM: Macular pucker following retinal surgery: A biomicroscopic study. Can J Ophthalmol 4:20–23, 1969.
11. Jaffe NS: Macular retinopathy after separation of vitreoretinal adherence. Arch Ophthalmol 78:585–591, 1967.
12. Maumenee AE: Further advances in the study of the macula. Arch Ophthalmol 68:151–165, 1967.
13. Wise GN: Preretinal macular fibrosis (an analysis of 90 cases). Trans Ophthalmol Soc UK 92:131–140, 1972.
14. Mills PV: Preretinal macular fibrosis. Trans Ophthalmol Soc UK 99:50–53, 1979.
15. Kleinert H: Primä{umlaut-a}re Netzhaufä{umlaut-a}ltelung im Maculabereich. Graefes Arch Clin Exp Ophthalmol 155:350–358, 1954.
16. Von Gloor B, Werner H: Postkoagulative und spontan aufretende internoretinale Fibroplasie mit Maculadegeneration. Klin Monatsbl Augenheilkd 151:822–845, 1967.
17. Noble KG, Carr RE: Idiopathic preretinal gliosis. Ophthalmology 89:521–523, 1982.
18. Yagoda AD, Walsh JB, Henkind P: Idiopathic Preretinal Macular Gliosis. Boston, Little, Brown, 1981.
19. Wise GN: Macular changes after venous obstruction. Arch Ophthalmol 58:544–557, 1957.
20. Hagler WS, Aturaliya U: Macular puckers after retinal detachment surgery, Br J Ophthalmol 55:451–457, 1971.
21. Lobes LA Jr, Burton TC: incidence of macular pucker after retinal detachment surgery. Am J Ophthalmol 85:72–77, 1978
22. Hirokawa H, Jalkh AE, Takahashi M, et al: Role of the vitreous in idiopathic preretinal macular fibrosis. Am J Ophthalmol 101:166–169, 1986.
23. Wise GN: Relationship of idiopathic preretinal macular fibrosis to posterior vitreous detachment. Am J Ophthalmol 79:358–362, 1975.
24. Iwanoff A: Beiträ{umlaut-a}ge zur normalen und pathologischen Anatomie des Auges. Graefes Arch Clin Exp Ophthalmol 11:135–170, 1865.
25. R . Sheard ,C. Sethi , Z. Gregor Acute macular pucker.Ophthalmology ,Vol 110 ,Issue 6 ,Pages 1178 – 1184
26. Optical coherence tomography in uveitis patients .Amer Journal of Ophthalmology ,Volume 130 ,Issue 5 ,Pages 669 - 670
27. Epiretinal membrane formation with internal limiting membrane wrinkling after Nd:YAG laser membranotomy in valsalva retinopathy.Kwok AK, Lai TY, Chan NR.
28. V. Levent, Berna , Özgül , Yusuf, Kocaeli Üniversitesi, T?p Fakültesi, Göz Hastal?klar? AD., Kocaeli Yard, Fakültesi, Göz Hastal?klar? AD., Kocaeli Prof. Dr Etiology and Vitreoretinal Surgery Outcomes in Epiretinal Membranes. Journal of Retina-Vitreous 2006, Volume 14, Number 3, Page(s) 193-196
29. JAHNClaus E. ; MINICHViktoria ; MOLDASCHELStefan ; STAHLBirgit ; JEDELHAUSERPhilipp ; KREMERGundula ; KRONMartina ; Epiretinal membranes after extracapsular cataract surgery. Journal of cataract and refractive surgery2001,vol.27,no5,pp.753-760.
30. Katira RC, Zamani M, Berinstein DM, Garfinkel RA Incidence and characteristics of macular pucker formation after primary retinal detachment repair by pars plana vitrectomy alone. Retina.2008 May;28(5):744-8.
31. EyyupKarahan,GulArikan,MeltemF.Soylev &AliOsman Saatci:Bilateral Idiopathic Epiretinal Membranes Associated With Multiple Peripheral Neurofibromas In A Young Adult:The Internet Journal of Ophthalmology and Visual Science. 2007;Volume5,Number1.
32. Vascularised epiretinal membrane associated with vasoproliferative tumour; Shankar, P. Bradshaw, S. E. Ang, A. Rennie, I. G. Snead, D. R. Snead, M. P; eye – London ophthalmological society of the united kingdom then royal college of ophthalmologists-2007, vol 21; number 7,1003-1004
33. Wise GN: Clinical features of idiopathic preretinal macular fibrosis. Am J Ophthalmol 79:349–357, 1975.
34. Wiznia RA: Natural history of idiopathic preretinal macular fibrosis. Ann Ophthalmol 14:876–878, 1982.
35. Allen AW Jr, Gass JDM: Contraction of a perifoveal epiretinal membrane simulating a macular hole. Am J Ophthalmol 82:684–691, 1976.
36. Barr CC, Michels RG: Idiopathic nonvascularized epiretinal membranes in young patients: Report of six cases. Ann Ophthalmol 14:335–341, 1982.
37. Messner KH: Spontaneous separation of preretinal macular fibrosis. Am J Ophthalmol 83:9–11, 1977.
38. Sumers KD, Jampol LM, Goldberg MF, Huamonte FU: Spontaneous separation of epiretinal membranes. Arch Ophthalmol 98:318–320, 1980.
39. Retinal distortion and cotton-wool spots associated with epiretinal membrane contraction, ARROYOJ. G. IRVINEA. R; Ophthalmology; 1995,vol.102,662-668.
40. Margherio RR, Cox MS Jr, Trese MT, et al: Removal of epimacular membranes. Ophthalmology 92:1075–1083, 1985.
41. Manschot WA: Persistent hyperplastic primary vitreous; special reference to preretinal glial tissue as a pathological characteristic and to the development of the primary vitreous. Arch Ophthalmol 59:188, 1958.
42. Wolter JR: Glia of human retina. Am J Ophthalmol 48:370, 1959.
43. Smith TR: Pathologic findings after retina surgery. In Schepens CL (ed): Importance of the Vitreous Body in Retina Surgery with Special Emphasis on Reoperations. St Louis, CV Mosby, 1960, pp 61–75.
44. Foos RY: Vitreoretinal juncture; Simple epiretinal membranes. Graefes Arch Clin Exp Ophthalmol 189:231–250, 1974.
45. Smiddy WE, Maguire AM, Green R, et al: Idiopathic epiretinal membranes. Ophthalmology 96:811–821, 1989.
46. Stern WH, Fisher SK, Anderson DH: Epiretinal membrane formation after vitrectomy, Am J Ophthalmol 93:757–772, 1982
47. Vinores SA, Campochiaro PA, Conway BP: Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthal Vis Sci 31:14–28, 1990.
48. Wilkins JR, Puliafito CA, HEe MR, et al, Characterisation of ERM using OCT. Ophthalmology 1996; 103;2142-51
49. Margherio RR, Trese MT, Margherio AR, Cartright K, Surgical management of vitreomacular traction syndromes. Ophthalmology 1989; 96: 1437-45
50. classification of epiretinal membranes based on findings by optical coherence tomography.mori keisuke,saito tamiya, ebisawa nobusuke,yoneya shin;Japanese Journal of Clinical Ophthal, vol.56;no.6;page.1005-1009(2002)
51. Machemer R: Die chirurgische entfernung von epiretina en makulamembranen (macular puckers). Klin Monatsbl Augenheilkd 173:36–42, 1978.
52. deBustros S, Thompson JT, Michels RG, et al: Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol 72:692–695, 1988
53. deBustros S, Rice TA, Michels RG, et al: Vitrectomy for macular pucker after treatment of retinal tears or retinal detachment. Arch Ophthalmol 106:758–760, 1988.
54. Ferencz JR, Nussbaum JJ, Richards SC, et al: Predictive variables in vitrectomy for idiopathic epimacular membranes. Submitted for publication.
55. Fisher YL, Shafer DM, Yannuzzi LA: Microsurgical management of macular epiretinal membranes (macular pucker). Dev Ophthalmol 5:122–130, 1981.
56. Haut J, Larricart P, Sfeir T, van Effenterre G: Treatment of epiretinal membranes: Ophthalmologica 183:190–196, 1981.
57. Mandelcorn MS, Liao R:Preretinal membranectomy in idiopathic preretinal macular fibrosis. Can J Ophth 18:321–324, 1983.
58. Margherio AR, Nachazel DP, Murphy PL, et al: The surgical management of epiretinal membranes. [Scientific exhibit] American Academy of Ophthalmology Annual Meeting, 1981. Ophthalmology 88(Suppl):82, 1981.
59. Rice TA, deBustros S, Michels RG, et al: Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology 93:602–610, 1986.
60. Pessin SR, Olk RJ, Grand MG, et al: Vitrectomy for premacular fibroplasia: Prognostic factors, long-term follow-up and time course of visual improvement. Ophthalmology 98:1109- 1114, 1991.
61. Wilkinson CP: Recurrent macular pucker. Am J Ophthalmol 88:1029–1031, 1979.
62. Margherio RR, Cox MS Jr, Trese MT, et al: Removal of epimacular membranes. Ophthalmology 92:1075–1083, 1985.
63. Pessin SR, Olk RJ, Grand MG, et al: Vitrectomy for premacular fibroplasia: Prognostic factors, long-term follow-up and time course of visual improvement. Ophthalmology 98:1109–1114, 1991.
64. Michels RG: Vitreous surgery for macular pucker. Am J Ophthalmol 92:628–639, 1981
65. Sawa M, Saito Y, Hayashi A, Kusaka S, Ohji M, Tano Y. Assessment of nuclear sclerosis after nonvitrectomizing vitreous surgery. Am J Ophthalmol 2001;132(3):356-62.