Introduction
Over the past few decades, the approach to treating primary rhegmatogenous retinal detachment (RRD) has undergone significant changes. Initially, scleral buckle (SB) was the standard method, followed by the occasional use of pneumatic retinopexy (PnR), and more recently, pars plana vitrectomy (PPV). This shift has primarily been driven by technological advancements rather than considerations of post-operative functional outcomes or the effectiveness of retinal reattachment.1
However, there is now a growing interest in ensuring both the integrity of reattachment and the resulting functional outcomes, spurred by the availability of multimodal imaging. The goal is not only to increase the success rate of retinal reattachment but also to optimize the reattachment process for the best possible functional results. One challenge observed with PPV is post-operative retinal displacement, also known as a low-integrity retinal attachment (LIRA)2–4. This displacement occurs due to the buoyant force of a large gas bubble, which can displace residual subretinal fluid beneath the thin, elastic retina during the final stages of surgery, especially when changes in head position occur. This stretching of the retina increases the risk of post-operative aniseikonia and/or metamorphopsia.2–4
History
In 1911, Ohm pioneered intravitreal air injection for treating rhegmatogenous retinal detachment (RRD), achieving success through innovative positioning of patients. Two decades later, Arruga combined intravitreal air injection with diathermy to aid subretinal fluid drainage. Rosengren in 1938 coined 'tamponade' and introduced intravitreal air bubbles to seal retinal breaks, but rapid bubble dissolution limited its adoption. Subsequently, the encerclage technique and intravitreal sulphur hexafluoride (SF6) improved outcomes for giant retinal tears.5
In the 1980s, Dominguez introduced modern pneumatic retinopexy (PnR) as an outpatient procedure, using SF6 to tamponade breaks and allow subretinal fluid reabsorption.5 Hilton and Grizzard coined the term pneumatic retinopexy (PnR) in 1986, popularizing the procedure.6 Clinical trials by P. Tornambe compared PnR with scleral buckle for RRD repair, demonstrating comparable primary reattachment rates and superior visual outcomes in macula off RRD cases.7,8 While scleral buckle remained preferred, R. Machemer revolutionized vitreoretinal surgery with pars plana vitrectomy (PPV) in 1971, which evolved over decades with gas tamponade and smaller-gauge systems.9,10
PnR indication and Contraindication
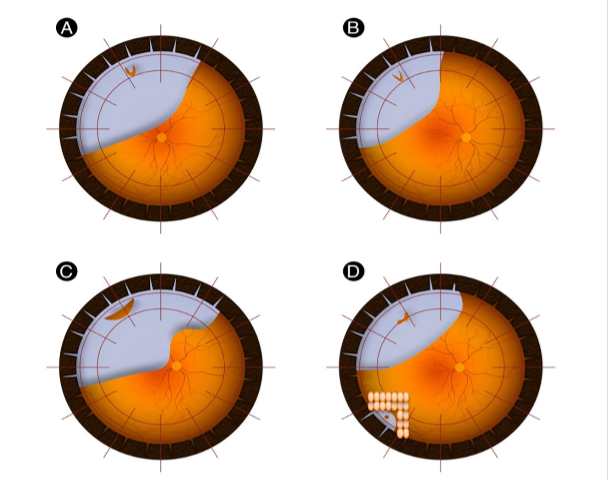
Table 1: Describes the various indications and Contraindications for PnR as per the PIVOT trial11
|
Indications For PnR |
Contraindications for PnR |
|
A single break or group of breaks no larger than 1 clock hour in detached retina (Figure 1A & B) |
Breaks within the inferior 4 clock hours in detached retina |
|
All breaks in detached retina above the 8 and 4 o’ clock meridian (Figure 1B) |
Proliferative vitreoretinopathy grade B or worse |
|
Breaks or lattices in the attached retina at any location (Figure 1D). |
Significant Vitreous Hemorrhage which obscures peripheral retinal examination |
|
Significant media opacity which obscures peripheral retinal examination |
|
|
Mental incapacity to follow post op positioning |
|
|
Physical incapacity to follow post op positioning |
Operative Technique
1. A comprehensive assessment of the peripheral retina is conducted through scleral depression to identify clock hours of detachment; size and clock hours of the break(s) or any other peripheral retinal pathology in the attached and detached retina; grade of PVR. A good preoperative evaluation is pivotal to the success of PnR.
2. Before proceeding with gas injection, it is crucial to perform laser retinopexy or cryopexy on all existing break(s) or peripheral retinal degeneration in the attached retina (Figure 1D).
3.Retinopexy: Choosing between Cryopexy and laser retinopexy depends on the surgeon's preference and the clinical situation. Laser retinopexy is preferred in cases of multiple, posterior, or large breaks. Cryopexy should be performed before aqueous tap and gas injection under subconjunctival anesthesia, while Laser retinopexy can be done 24-48 hours after gas injection if the break(s) is flat.
4. Pneumatic Technique: This is an in-office procedure performed under topical or subconjunctival anesthesia with strict aseptic measures. It can be done over the slit lamp or examination chair using proper light source. It can also be performed in a minor procedure room under a microscope.
A. Anterior Chamber Aspiration: We prefer a 1ml or tb syringe with a 30G or 27G, ½ inch needle and a speculum. Remove the plunger of the syringe and inject the needle around the inferior limbus over the iris (with the needle hub facing towards the cornea). Simultaneously place a sterile cotton bud or cue tip in the opposite quadrant to the tap site to stabilize the globe and put some pressure to dome the cornea which helps to protect the cornea from endothelial injury with the needle. Ideally, a minimum volume of 0.3 ml of tap is needed but if more aqueous comes without any risk of complications, we encourage that. Regardless of the gas chosen, an anterior chamber tap is a necessary standard procedure.
B. Gas Injection:
1. Type: Our preference is 100% Sulphur hexafluoride (SF6), ideally at 0.6 ml, due to its rapid expansion and shorter dissipation time. If a larger gas bubble or longer tamponade is needed, a second gas bubble, often SF6, can be added at the appropriate time. While 100% C3F8 can be an option at an ideal volume of 0.3 ml, its longer duration inside the eye may negate the advantages of an office-based procedure.
2. Volume: 1.2cc of expanded gas creates a 90-degree arc of contact, equivalent to 3 clock hours. Hence, for a break(s) less than or equal to 1 clock hour, the minimal gas required after expansion is 1.2cc, which corresponds to either 0.6ml of SF6 (100%) or 0.3ml of C3F8 (100%). Rarely, if the aqueous tap is less than 0.3ml, never inject less than 0.6cc of SF6 or 0.3cc of C3F8 for the reasons explained earlier. We can do repeat aqueous tap to stabilize the intraocular pressure. In case a higher volume of anterior chamber tap is obtained, add 0.1cc of SF6 or 0.05cc of C3F8 extra for every 0.1ml increase in aqueous tap. For instance, with 0.4ml of aqueous tap, inject 0.7cc of SF6 (instead of 0.6 cc) or 0.35cc of C3F8 (instead of 0.3cc).
3. Site: It is preferable to inject the gas away from large open breaks and bullous retina (though not mandatory). Tilt the head and globe so that the injection site is uppermost/highest, often in the superonasal or superotemporal quadrant, in line with our preference.
4. Technique: After topical or subconjunctival anesthesia and repeat aseptic precautions using a 3ml syringe with a 30G / 27G ½ inch needle and a lid speculum, inject the gas in the chosen quadrant. Insert the needle 6 to 8 mm into the eye to ensure it is well into the vitreous, directing it away from areas of highly bullous detachment. Withdraw it until 3 mm of the needle remains in the eye, ensuring the tip remains in the vitreous but is shallow enough to prevent multiple small bubbles ("fish eggs"). Inject perpendicular to the floor, aiming towards the center of the eyeball. Inject at a moderate pace to avoid the formation of fish eggs. Do not release the plunger until the needle is out of the eye to prevent gas reflux in the syringe. Immediately cover the site with a sterile cotton bud or cue tip to prevent subconjunctival gas reflux.
C. Check: Have the patient sit upright and check for perfusion of the optic nerve head, if it appears blanched, instruct the patient to face down for a while. This maneuver helps liquified vitreous to migrate into the anterior chamber via zonules. Recheck for central retinal artery patency, if it remains blanched or pulsatile along with the patient experiencing headache or eye ache then perform a repeat aqueous tap. Once perfusion is confirmed, check for the correct positioning of the gas bubble, i.e., within the vitreous and without subretinal or suprachoroidal gas or in the petit’s canal. Also, check for any fish eggs.
D. Head Positioning: Different physicians have varying preferences for positioning. Some opt for a "steam-roller" maneuver regardless of the macular status, while others position directly over the break. Our preference is to use the "steam-roller" maneuver in cases of macula-threatening RRD (Figure 1 B) or bullous RRD with medium to large tears (Figure 1 C). Otherwise, direct positioning over the break is suitable (Figure 1 A). While there is no definitive evidence to specify the minimum duration for a face-down posture, Bansal et al’s12stages of retinal reattachment suggest that a minimum of two to four hours face down should be followed, after which the head can be gradually tilted upright based on the break(s) location. Sometimes, when the margin of RRD is close to breaks or lattice degeneration in the attached retina, we adjust the positioning to prevent subretinal fluid from migrating to those areas (Figure D). Positioning must be strictly maintained for 10-14 days until cryopexy or laser retinopexy forms the scar.
5. Postoperative assessment: Some physicians prefer to review on Day 1 and some on Day 2 postoperatively. A comprehensive assessment of the peripheral retina is conducted through scleral depression to identify the following:
- It's crucial to examine the gas bubble's position, as it may migrate subretinally in the case of a large break.
- Is retina completely/partially attached or not: If partially attached check for any migrated or residual fluid and, if present, investigate the presence of an open break in that specific area. In case it’s not attached check for primary break and compliance of the patient.
- Evaluate the flatness of the primary break(s) and the surrounding area; laser retinopexy planning is only possible if the area is flat (in case cryopexy was not performed).
- Examine for secondary breaks that may have occurred after gas injection.
6. Laser Retinopexy: This is the most challenging aspect of Pneumatic Retinopexy and requires proficiency in indirect ophthalmoscopy and laser retinopexy. We typically prefer indirect laser retinopexy, using either a 20D or 28D lens, and always administer subconjunctival lidocaine anesthesia. Laser treatment can be performed with or without the gas in the field of view. The advantage of gas in the field is a minified view, however, a disadvantage is the potential for excessive thermal burns leading to retinal necrosis and hole formation, as gas has insulating properties that slow heat dissipation compared to liquid vitreous. To remove the gas from the field of view, tilting the patient's head can be effective before laser treatment of the break.
7. Follow-up evaluations: The frequency of these assessments depends on whether there is residual subretinal fluid or not. If there is no fluid, we perform reassessments at 1 week, 2 weeks, 1 and 3 months postoperatively. However, if any residual subretinal fluid is present, we conduct more frequent reassessments, approximately every other day, until the fluid is absorbed. During these evaluations, a thorough examination of the peripheral retina is conducted through scleral depression to determine the status of the gas bubble, retinal attachment, the condition of the primary break, the effectiveness of surrounding retinopexy, and to identify any new breaks.
COMPLICATIONS
1. Fish Eggs: The primary concern with fish eggs is the potential for subretinal gas if a tear is sufficiently large. The recommended approach to address fish eggs is to maintain a face-down position for a day to allow the gas to expand and merge the bubbles.
2. Suprachoroidal Gas: Like fish eggs, suprachoroidal gas typically has no major drawbacks, except for the possibility of inadequate intravitreal gas volume, which could lead to a failure of the pneumatic retinopexy (PnR) procedure. Additional gas can be added on day 2 or 3 if it's felt that the intravitreal volume is insufficient.
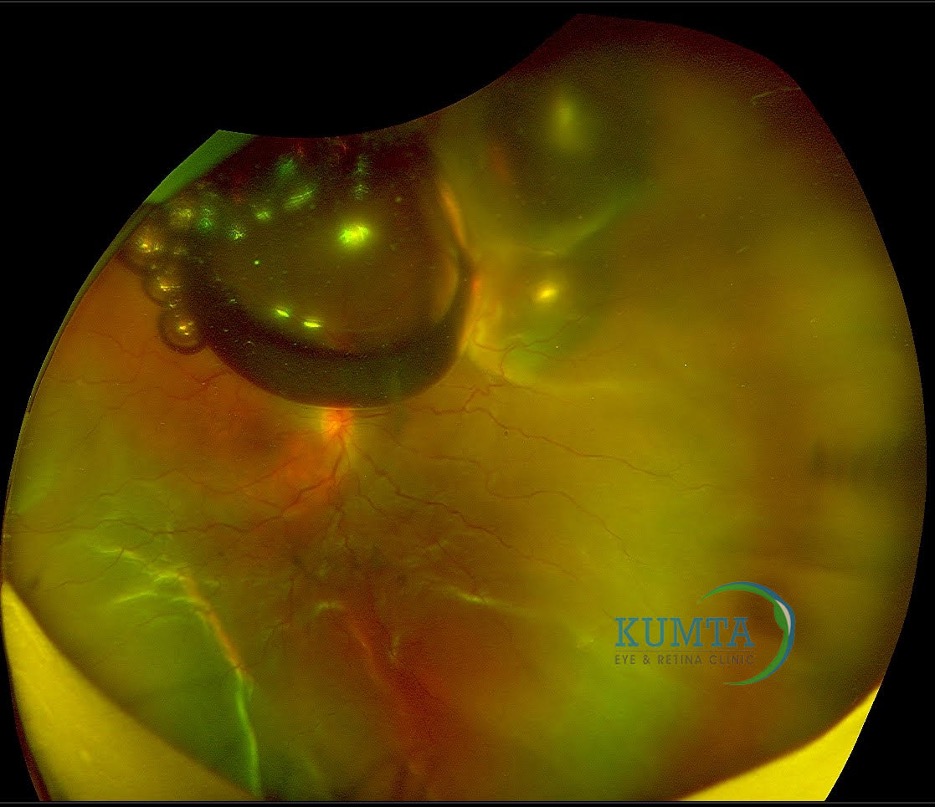
3. Subretinal gas: When a gas bubble gets trapped beneath the retina, it can cause the detached retina to appear like a pearly, dome-shaped refractile sheen (Figure 2). Treatment involves gently massaging the bubble back toward the retinal break using scleral depression and appropriate positioning. If this proves ineffective or if there is a significant amount of subretinal gas, surgical removal may be necessary. A small amount of subretinal already expanded gas can be closely observed.
4. Gas in petit space /canal of petit: Donut/Bagel/Sausage Sign with limited mobility of gas. Once confirmed immediately prone position for 24 hours, with buoyancy gas will seep into the vitreous. In case gas doesn’t move to vitreous space then need to plan for an urgent pars plana vitrectomy.
5. Elevated break on Day 1 or 2 postoperatively: In cases where the retinal break remains elevated shortly after the procedure, it's important to reassess the patient's compliance with the recommended head positioning and their understanding of it. Reinforce the importance of proper positioning and reevaluate on Day 3 postoperatively. If the break is still elevated, the issue may be related to either compliance or the size of the gas bubble. Inadequate gas bubble size may require a second gas bubble, while non-compliance necessitates a discussion with the patient about the critical nature of positioning in retinal reattachment.
6. Migrated or Residual Subretinal Fluid: Frequently, on postoperative days 1 or 2, we observe the migration of fluid to the attached part of the retina, which may raise concerns about the effectiveness of Pneumatic Retinopexy (PnR). However, this phenomenon primarily occurs due to the buoyant force exerted by the gas bubble, as documented by Lee et al.13 The presence of residual subretinal fluid following PnR is not uncommon. Residual Subretinal Fluid can persist for several months and, in rare cases, up to a year, without adversely affecting the final visual acuity at the one-year mark.14 What's crucial in these situations is the need for careful and repeated assessments to confirm the absence of any open breaks in the migrated or residual subretinal fluid area and monitoring of the fluid level. Ensure it is either resolving or remaining stable, without any signs of increasing. In the rare event of a fluid increase, it may indicate the presence of an occult break, and further discussions between the patient and the surgeon are necessary to determine whether additional gas injection or pars plana vitrectomy (PPV) with or without scleral buckling (SB) is warranted.
7. New Retinal Break: Historically higher rates of new breaks have been reported, but in the author’s personal experience they haven’t noted many cases of new breaks, or can happen due to late secondary posterior vitreous detachment.
FAILED PnR
Authors classify PnR as unsuccessful when it necessitates further intervention, such as PPV with or without SB, for a persistent or recurrent RRD. PnR is typically not considered a failure when additional rescue measures are employed within the first one to two weeks, such as a second gas bubble or retinopexy. Here are some crucial time points and recommendations for managing a PnR procedure that is not progressing as expected:
Day 0-2:
1. If there is a subretinal gas, you can attempt the aforementioned measures or consider immediate planning for PPV+/-SB.
2. If the primary break remains elevated, reinforce proper head positioning.
3. In the case of a new break, assess its location (attached or detached retina) and its effect on new subretinal fluid recruitment before planning your approach.
Day 3:
1. If the break remains elevated, there are two potential reasons to consider: either the gas bubble is insufficient, or the patient is not complying with the recommended head position. If the issue is related to the gas bubble, you should contemplate using a second gas bubble, employing the same procedure as the initial one.
2. For a new break, determine its location and impact on fluid recruitment before deciding on the next steps.
Day 7-14:
1. If the bubble starts to shrink and the break is lifted again (though this is not common), discuss with the patient the choice between repeat PnR and PPV, considering the advantages and disadvantages of each.
2. For a new break, assess its location and effect on fluid recruitment before planning your strategy.
3. In the event of a new RRD occurring in different clock hours, weigh the pros and cons of repeat PnR (if the break is in the superior 8 clock hours) versus PPV+/-SB.
Week 2:
If there is recurrent or new RRD in different clock hours, discuss with the patient the pros and cons of repeat PnR (if the break(s) are in the superior 8 clock hours) versus PPV+/-SB.
Conclusion
Pneumatic retinopexy has become an important surgical technique in the modern era of retinal surgical management for RRD. Despite its limitations, multiple clinical studies have clearly shown important and favorable features of PnR, and established its continued important role in the armamentarium of the retinal surgeon for managing an RD. The retinal surgeon should carefully consider all preoperative ocular features unique for each individual eye with an RD, modify his or her surgical techniques accordingly, and strive for the best possible anatomic and visual outcome for each patient.
References
1. Muni RH, Lee WW, Bansal A, Ramachandran A, Hillier RJ. A paradigm shift in retinal detachment repair: The concept of integrity. Prog Retin Eye Res. Published online October 2022:101079. doi:10.1016/j.preteyeres.2022.101079 2. Francisconi CLM, Marafon SB, Figueiredo NA, et al. Retinal Displacement after Pneumatic Retinopexy versus Vitrectomy for Rhegmatogenous Retinal Detachment (ALIGN). In: Ophthalmology. Vol 129. Elsevier Inc.; 2022:458-461. doi:10.1016/j.ophtha.2021.12.007 3. Brosh K, Francisconi CLM, Qian J, et al. Retinal Displacement following Pneumatic Retinopexy vs Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment. In: JAMA Ophthalmology. Vol 138. American Medical Association; 2020:652-659. doi:10.1001/jamaophthalmol.2020.1046 4. Mason RH, Minaker SA, Marafon SB, Figueiredo N, Hillier RJ, Muni RH. Retinal displacement following rhegmatogenous retinal detachment: A systematic review and meta-analysis. Surv Ophthalmol. Published online 2022. doi:10.1016/j.survophthal.2022.01.002 5. Fernández‐Vega González A, Muni RH. The history of pneumatic retinopexy: have we come full circle? Acta Ophthalmol. 2022;100(1):118-120. doi:10.1111/aos.14876 6. Hilton GF, Grizzard WS. Pneumatic Retinopexy. Ophthalmology. 1986;93(5):626-641. doi:10.1016/S0161-6420(86)33696-0 7. Tornambe PE, Hilton GF, Brinton DA, et al. Pneumatic Retinopexy: Two-year Follow-up Study of the Multicenter Clinical Trial Comparing Pneumatic Retinopexy with Scleral Buckling. Ophthalmology. 1991;98(7):1115-1123. doi:10.1016/S0161-6420(91)32168-7 8. Tornambe PE, Hilton GF, Poliner LS, et al. Pneumatic Retinopexy: A Multicenter Randomized Controlled Clinical Trial Comparing Pneumatic Retinopexy with Scleral Buckling. Ophthalmology. 1989;96(6):772-784. doi:10.1016/S0161-6420(89)32820-X 9. Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813-820. 10. ECKARDT C. TRANSCONJUNCTIVAL SUTURELESS 23-GAUGE VITRECTOMY. Retina. 2005;25(2):208-211. doi:10.1097/00006982-200502000-00015 11. Hillier RJ, Felfeli T, Berger AR, et al. The Pneumatic Retinopexy versus Vitrectomy for the Management of Primary Rhegmatogenous Retinal Detachment Outcomes Randomized Trial (PIVOT). Ophthalmology. 2019;126(4):531-539. doi:10.1016/j.ophtha.2018.11.014 12. Bansal A, Lee WW, Felfeli T, Muni RH. Real-Time In Vivo Assessment of Retinal Reattachment in Humans using Swept-Source Optical Coherence Tomography. Am J Ophthalmol. 2021;227:265-274. doi:10.1016/j.ajo.2021.02.013 13. Lee WW, Ramachandran A, Hamli H, Escaf LC, Bansal A, Muni RH. Immediate subretinal fluid displacement from the buoyant force of a small gas bubble in pneumatic retinopexy: Insights into the potential mechanism of retinal displacement following retinal detachment repair. doi:10.1097/ICB.0000000000001187 14. Bansal A, Lee WW, Sarraf D, et al. Persistent subfoveal fluid in pneumatic retinopexy versus pars plana vitrectomy for rhegmatogenous retinal detachment: posthoc analysis of the PIVOT randomised trial. British Journal of Ophthalmology. Published online August 11, 2022:bjophthalmol-2021-320981. doi:10.1136/bjo-2021-320981

