INTRODUCTION
Pars plana vitrectomy as a technique has revolutionized retinal surgery since its advent and initial report by Machemeret al1. It allowed the removal of traction by an internal method, essential in retinal detachment procedures, as well as provided an active management modality for vitreous haemorrhage and opened the door for surgical intervention in a myriad of retinal pathologies.
Since that time, the evolution of vitrectomy surgery has seen experimentation and implementation of smaller surgical instruments aimed at greater functionality and minimalization of ocular trauma. The basis of a sutureless pars plana sclerotomy was to stabilize intraocular pressure (IOP) during surgery, with a truly closed system, as well as reduce surgical time by removing the need for sutured wound closure. Wound and suture related complications such as leakage, irritation, and scleral pigmentary changes could also be avoided. Concerns regarding wound competence in a sutureless procedure have seen the modification of the conventional straight incision to such techniques as angled, beveled, oblique, and scleral tunnel incisions
HISTORY:
Von Graefe was the first one to invade the sacrosanct vitreous in 1863. The earlier period of vitreous surgery began in 1889, extended through the 1960s and was the domain of relatively few courageous surgeons. These ophthalmologists approached the vitreous pathology either through the pars plana or more commonly through the anterior segment.
David Kasner in 1968 removed all the diseased vitreous in case of amyloidosis of vitreous by his so-called ‘Open Sky’ Vitrectomy. In doing so he not only opened the cornea but also opened the sky of ocular surgery for ophthalmic surgeons. But this open sky route had its inherent problem of making the eye aphakic, large instruments obscuring the view of the operating plane and open eye leading to global collapse. The earlier technique of radical vitreous surgery included the replacement of diseased vitreous with numerous substances including silicon oil, collagen, hyaluronic acid, cerebrospinal fluid, cadaver vitreous and synthetic polymers.
It took almost 100 years, ever since the invasion of vitreous by various workers to develop a machine that could cut and aspirate the vitreous. Dr. Robert Machemer began working on vitreous surgery by introducing a rotating drill in the eggshell by removing the egg white. On April 20, 1970, first closed Vitrectomy was performed on a human eye. As Machemer puts it, “This man had been blind for years; he had a vision of finger counting at 2 feet, slight cataract and his vitreous was opaque. We were very lucky to select a patient with a condition that we know today to be easiest to treat, just hemorrhage. We were also lucky that we did not have any complications. One can imagine the enthusiasm that we had when the first operation was a full success and the patient could see 20/50.”
In 1971, Machemer et al described the use of a 17-gauge vitreous cutter, with a diameter of 1.5 mm through a 2.3 mm scleral incision. This instrument, the vitreous infusion suction cutter (VISC), consisted of an inner and outer tube. The outer tube was stationary with an opening, inside which, was the rotating opening of the inner tube with sharp edges. Suction was applied to the inner tube to draw vitreous into the openings and the rotating sharp edge would cut the material. The instrument was connected to a rheostat to alter rotation speed, an infusion system, and a syringe that allowed the manual application of suction. The fibreoptic system to provide endoillumination was introduced in 1972, and was added as a sleeve around the tube.2 All these procedures were 2 port procedures as infusion and cutter/suction function were done through a single probe.
The 2 port approach was modified in 1974, with the introduction of a 20-gauge vitrector (0.9 mm ) 3. This was the origin of the three-port, pars plana sclerotomy system that became the gold standard in vitrectomy surgery. It involved the creation of three access ports with a 1.4 mm linear sclerotomy. This was undertaken with a myringo-vitreal-retinal (MVR) blade. One port had an infusion line sewn into place, while the remaining two were utilized for the introduction of a light source and a vitreous instrument such as a cutter. At the completion of the procedure, these ports were traditionally closed with an absorbable suture.
Conor O’Malley and Ralph Heinz also developed a lightweight, reusable, bellows-driven, pneumatic, axial cutter driven by the Ocutome 800 console (Berkley Bioengineering, 1972). Gholam Peyman developed the electric solenoid driven axial (guillotine) cutter at about the same time R. Kloti in Europe developed a three-part system with an electric cutter.
The introduction of endophotocoagulation by Charles Schepens allowed retinopexy, hemostasis, and pan-retinal photocoagulation without corneal or iris damage. His first used the Zeiss xenon source. Subsequently, Maurice Landers and Jay Fleischman with Chares Schepens simultaneously and independently developed endophotocoagulation systems using an argon laser source. Later Yasuo Tano developed the near-IR diode laser source and finally, Alcon and Iridex developed 532 nm, diode-pumped sources.
In 1996, Chen 4 described a technique for creating a self-sealing, pars plana sclerotomy. This involved an initial scleral incision based 6 mm posterior to the limbus, creating a scleral flap that was theoretically self-sealing. Kwoket al5 described a variation on this method with an initial radial incision, still placed 3-4 mm behind the corneoscleral limbus. They used a 20-gauge round body hypodermic needle rather than an MVR blade.
De Juan and Hickingbotham 6 devised and introduced a range of 25-gauge instruments in 1990 for use through conventional sclerotomies. However, it was only in 2002, with the advent of the microcannulae array, that the 25-gauge transconjunctival sutureless vitrectomy (TSV) system was introduced by Fujiiet al7. This was followed by the introduction of a 23-gauge system by Eckardt in 2005. 8
Initially, both 23- and 25-gauge systems were available with a limited gamut of intraocular instruments. However, as the techniques rapidly became widely utilized, almost all intraocular instruments have been developed and made available for sutureless vitrectomy systems.
Objectives of Vitrectomy
-
Clearing the media & access to the diseased retina,
-
Creating a space for internal tamponade
-
To cut Vitreo-Retinal Membranes
-
To release vitreoretinal traction
-
Retinal manipulation and reattachment
-
Removal of tissue or foreign material & to obtain a vitreous biopsy
Indications for Vitrectomy9
There is rapid innovation in the technology of vitreous surgery and along with it the indications of Vitrectomy keep on increasing/changing.
These indications can be broadly divided into anterior Segment indications and posterior Segment indications.
Anterior segment1. Opacities of the media
2. Anterior segment reconstruction as for in Adherent leucoma 3. Lens surgery
4. Complications due to Vitreous in Anterior Segment.
5. Miscellaneous conditions such as epithelial cysts, epithelial downgrowth / ingrowth etc. |
Posterior SegmentThese can be divided into diagnostic and therapeutic 1. Diagnostic indications
2. non-resolving vitreous opacities like vitreous hemorrhage, amyloidosis, membranes
4. Vitreous opacities like hemorrhage, membrane,amyloidosis
6. Retinal Detachment:
7.Epiretinal Membrane |
Vitrectomy
There are four principles to removing vitreous in a controlled fashion. First, do not pull the vitreous too close to the probe. Second, do not pull vitreous too far up the probe. Third, remove the vitreous in tiny pieces. Fourth, use as low a flow as possible. Regardless of the cut-rate, vacuum, or duty cycle selected, it is important to maintain these four principles to avoid putting traction on the retina and cutting unintentionally.
Vitrectomy can be discussed under the following headings:
-
Viewing system
-
Machine
-
Vitreous cutters
-
Accessaries
-
Simple vitrectomy
-
Advances
Viewing System
The viewing system is essential as we cannot directly view the posterior segment of the eye through the operating microscope. This occurs because of the refraction of the rays of light from the fundus by the refractive elements in the eye.The viewing system can be either:
1. Contact system:They neutralize the refractive power of the eye. These can be either conventional lenses like handheld lenses (fig 1A), sew on lenses, self-stabilizing lenses or wide-angle lenses. (fig. 1B)


Fig:1A Hand held irrigating contact lens . Fig 1B: Contact Lenses with Fixation Ring
2. Non contact system:Non contact systems are
i. BIOM: Binocluar Indirect Ophthalmo Microscopy. Incorporates the principles of indirect ophthalmoscopy in the operating microscope. (fig 2)
ii. EIBOS: Erected image binocular ophthamomicroscope (fig 3)
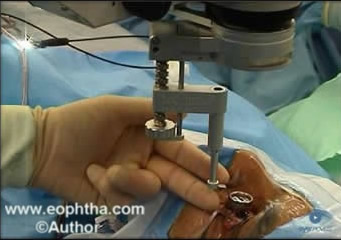

Fig:2 BIOM Fig:3 EIBO
Machine (FIG 4)
The modern Vitrectomy machine has the following components
-
Dual illumination module
-
High-frequency diathermy module
-
Air module
-
Vitrectomy module
-
Viscous fluid injection and extraction
-
Fragmentation module
-
Extrude mode

Fig 4: Vitrectomy Machine
1. Dual illumination module: The machineshould provide simultaneous use of two or three illumination ports. The light source is generally a Halogen bulb and an automatic switch should occur to the second lamp when the first lamp fails. There should be an integrated heat protection filter for cold light and UV/IR filtering.
2.High-frequency diathermy module: -Machines should have diathermy for endo and exo diathermy for selective burn placement on retina and sclera. Diathermy function should also be available during the priming function to avoid any delays.
3. Air module: -Air module provides an automatic air infusion system. Air is delivered to the tubing set through a 0.22 mm filter to assure sterility.
4. Vitrectomy Mode: -A standard high-speed cutter has cutting frequency of up to 2500 cpm. High speed cutting offers reduced traction and increased stability while working close to retina. The machine should support both pneumatic and electric drive for pneumatic and electric vitrectomies. It should be usable in single cut, fixed and linear cutting control. New horizontal cutting probes have a radial reciprocating action to minimize turbulence or fluttering of tissues (cutting blade moves from left to right across the port). Latest new vitrectomy machine by alcon The Constellation ca cut upto a frequency of 5000cpm.Three different vitrectomy modes exist: proportional, 3D(dual dynamic drive) and momentary.
3D Technology:This allows the operator to simultaneously change cut rate and vacuum which provides way to easily change parameters as needed throughout the surgery. Vacuum can be set to start at low level and rise to max at full footpedal depression,while cutting rate can be set to start at its max setting and decreased as footpedal is depressed.It allows more cutting of vitreous while doing core vitrectomy and fine cutting without much pull when working near retinal surface.
5. Viscous Fluid Injection and Extraction: -Pneumatic drive for Viscous Fluid Injection with Fixed or Linear controlled pressure injection with a pressure range should be provided for injecting silicon oil if required.For extraction, there is facility for linear vacuum controlled viscous fluid extraction.(fig 5)

Fig 5
6. Fragmentation: -The Fragmentation mode provides smooth, efficient ultrasonic fragmentation and vacuum using a lightweight hand piece connected to the surgical system with an electric cable and vacuum tubing.
Four different fragmentation modes exist:
-
Linear,
-
Momentary,
-
3D Fragmentation and
-
Fixed Fragmentation.
These multiple modes help accommodate surgeon preference. The Linear Fragmentation mode is specifically designed to control and emulsify subluxated lenses, and the new 3D Fragmentation mode allows simultaneous control of ultrasonic power and vacuum.
7. Extrude:
It is an active suction useful for PVD induction, suction of subretinal fluid, non-clotted blood, fluid-air exchange. (fig 6)

Fig 6: Extrusion Cannulas
Modern Vitrectomy Machines also offers following advantages:
Air-fluid Exchange
There should be control of preset and actual infusion pressure of air in pressure range of 5-95 mmHg for air fluid exchange.
Automated Infusion Pressure
Digital infusion pressure provides immediate response to increase or decrease IOP and gives the surgeon precisely controlled pressurized infusion for chamber stability. The surgeon-elevated infusion feature provides both ease-of use and control of infusion pressure via the foot pedal to instantly control intraocular bleeding. Digital control eliminates manual raising and lowering of the IV pole, which is required with traditional gravity infusion method.
It is important for maintaining the globe volume and preventing hypotony during intraocular manipulation.
Infusion fluid to the eye is delivered by an infusion cannula anchored to the eye in the inferotemporal quadrant. Infusion has to be switched off during entering and exiting through the sclerotomy in order to prevent the fluid from seeping subretinally or into the suprachoroidal space. In full function system, the multipurpose probe has facility for infusion along with other functions.
Scissors
The Scissors mode provides cutting capability using pneumatically or electrically powered scissors controlled by the foot switch in three modes: Proportional, Multi-cut and MPC. Automated MicroScissors and Forceps facilitate segmentation, delamination and en block scissors techniques
Vitreous Cutters
Electrodynamic cutters are heavy, becomes hot, causing fatigue and exacerbating tremors. (fig 7)
Pneumatic cutters are lighter, so they cause fewer tremors and are cheap (fig 8)
The Vitrectomy cutters are basically of three types
-
Cutters using rotating mechanism
-
Cutter using oscillating mechanism: - This type of cutter is considered to be superior to the first one as it has less shearing effect. This type was popularized by Peyman and co-workers.
-
The Guillotine type cutters: - It has an outer tube which is fixed and has a opening through which vitreous is aspirated. The inner tube slides across the port thus cutting the vitreous.(fig 9)
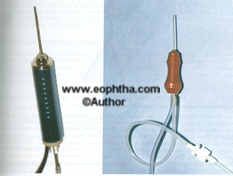
Fig 7:Electric and pneumatic cutters

Fig 8: Different types of Cutters
Illumination
Good illumination is absolutely necessary for vitreous surgery. The illumination available should ensure visualization but at the same time it should be non-toxic to the retina.The illumination is available either by external source or endoillumination. External illumination is provided through the operating microscope and is usually employed for anterior segment manipulations. Intraocular illumination was found to be superior to coaxial or other external systems because it provided bright illumination that was nearly free of disturbing reflexes. This reflex-free endoillumination for vitreous surgery is provided separately through a different source by a fibre optic system. This can either be incorporated in the sleeve of full function probe or be used as a separate fibre optic terminal apart from the vitreous instruments. In the former the tip diameter becomes bigger and the illumination is in fixed relation between the instrument and the emitted light. The latter has some advantages like the light-pipe retina distance can be adjusted to prevent light induced retinal damage and the endoilluminator can be used as a second instrument for bimanual manipulations.
Surgical Steps:
1. Peritomy:The conjunctival incision is performed at the limbus both nasally and temporally, to facilitate the placement of vitrectomy ports. Relaxing incisions can be placed to prevent the ripping off the conjunctiva during the remainder of the procedure. (fig 10)
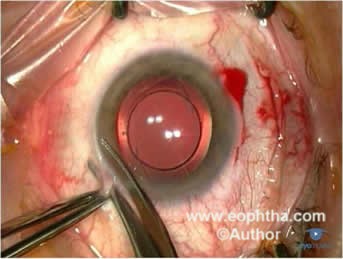
Fig 10:Peritomy
2. Viewing system:If a sew-in contact lens viewing system is to be used, a suture is placed at 3 and 9 o’clock positions near the limbus to facilitate placement of the lens ring. We use 6-0 vicryl for this purpose. Spatulated needle should be used for this purpose as it reduces the risk of perforation. Wet field cautery is performed throughout the area of exposed conjunctiva to reduce subsequent bleeding and oozing during the procedure.
3. Cannula:Infusion cannula is used to deliver the BSS into the vitreous cavity. MVR (microvitreoretinal) blade is used to create sclerotomy It is placed in the infero-temporal quadrant through sclerotomy made 3.5-4 mm posterior to limbus in phakic and 3-3.5mm posterior to limbus in aphakic or pseudophakic eyes. The sclerotomy should perforate the pars plana without causing damage to lens or retina. The length of the infusion cannula should be adequate to deliver the solution into the vitreous cavity while minimizing the risk of hitting the lens and retina during manipulations. 4mm infusion cannula is used for standard pars-plana vitrectomy. Longer cannulae may be employed with aphakic or pseudophakic patients or in patients with high myopia or peripheral retinal elevations such as in choroidal detachments in order to reduce the risk of cannula entering the subretinal space. 2mm cannula is sometimes employed to reduce the risk of lens damage. The cannula is held in place with 6-0 vicryl suture. After the cannula is sewn to the sclerotomy surgeon directly visualizes the position of the cannula in the vitreous cavity before turning it on. (fig 11a and 11b)
| Remember: infusion cannula is first to go in and last to come out. |
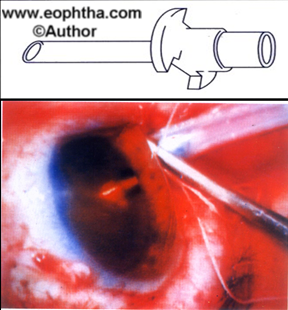
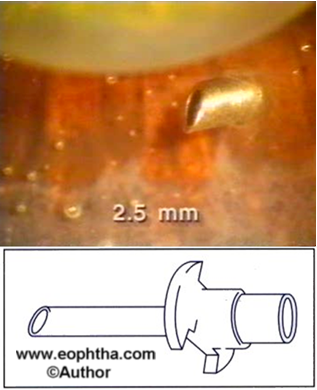
Fig 11A & 11B: Infusion Cannula
4. Instrument ports:Sclerotomy sites similar to that for cannula are made in superonasal and superotemporal quadrants 4 mm posterior to limbus in phakic and 3-3.5mm posterior to limbus in aphakic or pseudophakic eyes. These sites should be ideally 160° apart to facilitate manipulation in the vitreous cavity.
Core Vitrectomy:The goal of the vitrectomy is to remove the centrally formed vitreous first and then to move the vitrectomy gradually towards the retinal periphery so that a complete vitrectomy may be performed.
Incision of the central vitreous gel is performed initially. A light pipe is introduced through one of the port sclerotomies. Vitreous cutter is introduced through the other sclerotomy. The light pipe should be kept away from the cutter so that the tip of the cutter is well illuminated.
Once the instruments are in the mid-vitreous cavity, the surgeon depresses the foot pedal to begin aspiration and cutting. The cutting port on the vitreous cutter should face the vitreous to be cut, so that the surgeon visualise the cutting port at all times. The instruments are held steady in the the central vitreous cavity so that the vitreous is allowed to come to the vitreous cutter. Excessiove movement of the instruments in the vitreous cavity should be avoided as it will lead to peripheral retinal tears. When the vitreous appears to stop its migration towards the cutter, the cutter is advanced posteriorly to engage any vitreous. Scleral depression by the assistant permits trimming of the vitreous towards the peripheral vitreous base.
Removal of the posterior hyaloid
Extreme caution should be exercised while removing the posterior hyaloid. Any undue traction on the hyaloid will be transmitted to the vitreous base and can result in retinal and detachment. Posterior hyaloids is typically engaged in the peripapillary region where the potential for damage to the retina is less. Intra vitreal triamcinolone can be used for better visualisation of cortical vitreous and aids in complete hyaloid removal.
Vitreous substitutes:
As discussed earlier various substances have been tried in the past to replace the vitreous after vitrectomy. Vitreous should be replaced by some substitute to: -
1. Tamponade the retina
2. Replace the opaque vitreous with an optically clear media
3. Replace the vitreous volume
4. Expand the vitreous volume
The commonly used vitreous substitutes are
-
Gas:Air, SF6, C3F8 are the most commonly used gases
-
Saline
-
Silicon Oil
-
Perfluorocarbon liquids
-
Retinal tacks
Recent advances
In the ever evolving vitreous surgery there are srides of advances being made in the development of vitreous cutters, viewing systems, peeling instruments, vitreous substitutes.
One of the principles that guide the development of a surgical procedure is the desire for lesser invasive approaches that achieve similar if not better outcomes. Reduction in the incision size leads to minimization of tissue trauma and reduction in the post-operative convalescence period. The transition from conventional extra capsular surgery to phacoemulsification has shown a resultant decrease in surgical time, less post-operative inflammation and faster recovery. A similar analogy lies in the development of small gauge vitrectomy systems, which shorten the operating time, obviate the need of sutures (and hence suture related inflammation) and remarkably reduce damage to the tissue.
Small guage trans-conjunctival sutureless vitrectomy:(Fig 12)

Fig 12: 23 G and 25G cutters
Transconjunctival sutureless vitrectomy consists of a 23-g or 25-g microcannular system and a wide array of vitreoretinal instruments specifically designed for this operating system. The microcannula is a thin walled tube about 4mm in length. It has a collar in the extraocular portion which can be grasped with forceps to manipulate the micro-cannula. The sharp tipped insertion trocar forms a continuous bevel with the microcannula. This is made so to allow ease of entry through the conjunctiva. The 25-g infusion cannula consists of a small tube that fits securely and can be directly inserted into the cannula in the inferotemporal quadrant. A wide array of vitreoretinal microsurgical instruments complying with the 25-g standards has been designed. These include vitreous cutters, illumination probes, intraocular forceps, microvitreoretinal blades, tissue manipulator, aspirating picks, aspirators, soft-tip cannulas, curved scissors, extendable curved picks, intraocular laser probes, and diathermy probes.
Technique:Small guage vitrectomy is usually performed under local anaesthesia. It can also be performed under topical anaesthesia with light sedation. After thorough aseptic preparation the The microcannulas are inserted through the conjunctiva into the eye by means of a trocar. Insertion is accomplished by first displacing the conjunctiva laterally by approximately 2 mm. An initial oblique tunnel and then a perpendicular tunnel are made parallel to the limbus through the conjunctiva and sclera, thus creating a self-sealing wound.(Fig 13)
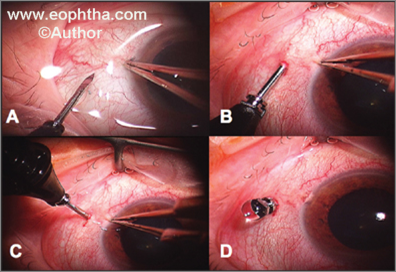
Fig13: Small Gauge Technique: Insertion of microcannulas
At the completion of the surgery, the microcannulas are simply removed by grasping the collar and withdrawing, with the assessment of intraocular pressure and wound sites for any possible leak. (fig 14)In 23 gauge system vitreous cutters have been improved with the placement of the cutter opening nearer to the end of the probe, which allows for a closer vitreous shave. This increases the safety near the retina.
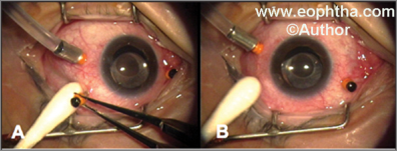
FiG 14: Removal of microcanula
Cutters:In the recent past, the concept of multi-function instruments has been revisited primarily to accomplish bimanual dissection of tissue that minimizes iatrogenic problems during operations and avoids a fourth sclerotomy. These multi-function instruments are available in several combinations such as illumination and forceps or illumination and scissors or infusion and illumination or suction and diathermy
To minimize the pull on the retina while removing vitreous Jelly, new high-speed cutters with 2000-5000 cuts per minute are available. Vitreous shaver is yet a new design of vitreous cutter that has two small openings at its tip, which prevents the retinal being sucked into the probe. The curved vitreous probe allows removal of the tissue from the opposite quadrant (180-degree away) without causing posterior lenticular touch; it also avoids the need to exchange instruments from one hand to the other. For pediatric patients undergoing vitreous surgery, new instruments with much smaller diameter of 0.6 mm (23 gauge) have been designed. These are especially useful while performing pars plicata vitrectomy or lens sparing vitrectomy in premature infants with advanced retinopathy of prematurity.
Viewing system:Besides operating microscope, vitreous surgery requires contact lenses and illumination to visualize intraocular structures. Availability of the wide-angle viewing system gives a panoramic view of the fundus; it permits a better control during complex surgical steps. Bullet light-pipe providing wide-angle illumination is used in conjunction with the wide-angle viewing system. Sometimes a xenon arc light source is preferred because of its bright, white illumination that gives a better contrast.
Temporary keratoprosthesis are also used to provide wide-angle viewing in those challenging cases where penetrating keratoplasty is performed along with vitreous surgery.
Vitrectomy endoscope that can be introduced through a very small incision inside the eye is yet another advance in vitreoretinal surgery.
In those eyes that are refractory to dilatation, disposable iris retractors mechanically retract the iris and thus aid in adequate visualization during surgery.
Peeling Instruments:A large number of peeling instruments such as membrane spatulas or hooks or forceps or scissors allow the surgeon to remove or peel the delicate membranes from the surface of the retina with least possible tissue trauma. The latest development includes the diamond-dusted forceps to provide a better grip, internal limiting (ILM) peeling forceps that holds this thin membrane and erbium laser that allows removal of the tissue layer by layer with no damage to underlying retina.
Macular surgery set:With a surge of interest in macular surgery, a separate set of macular instruments has been developed. These instruments are angled subretinal pick, subretinal infusion cannulas, subretinal forceps and scissors. Most of these instruments are 130-degree angulated and tapered to 0.305 mm (36-gauge) at the tip though the shaft diameter remains 20-gauge. This design helps the surgeon to create a retinotomy away from the center of the macula in the horizontal meridian.9
Latest Advancement in vitrectomy technology:
The CONSTELLATION Vision System (fig 15)(Alcon Laboratories, Inc., Fort Worth, TX) is an integrated vitreoretinal system that not only has advanced cutting capabilities, but also highly efficient flow and intraocular pressure control, an embedded 532 nm thin-disc laser system, and intelligent efficiency features that improve every aspect of a vitrectomy procedure.
It has a newly designed ULTRAVIT pneumatic probe that not only operates on a variable duty cycle, but adds the ability to control the bias of the cycle to keep it primarily open or primarily closed. This allows ability to cut at much higher rates and that duty cycles
are easily controllable. The surgeon can keep the port biased open, biased closed, or even biased. The working area is larger because of the ability to adjust duty cycle for a given cut
rate and as a result, the surgeon has the ability to operate in any location efficiently.
Also, one of the challenges that surgeons have faced is controlling intraocular pressure (IOP) in relation to the pressure of the fluid that is infused through the cannula during surgery. New integrated infusion pressure and IOP control system,which represents a significant advancement in safety for vitrectomy. The IOP control, automatically adjusts for the infusion tubing and pressure drop that occurs when fluid is flowing through the cannula by measuring and checking the infusion cannula and tubing resistance during priming. With the CONSTELLATION, the IOP is maintained to within ±2 mm Hg of the surgeon’s setpoint.

Complications
As with any surgery, vitrectomy has risks. Cataract, retinal detachment, high intraocular pressure, bleeding in the eye, and infection are among the possible complications. Cataract is the most frequent complication of vitrectomy surgery. Many patients develop a significant cataract within the first few years after vitrectomy.
The complications in vitreous surgery although uncommon in the present day surgery do occur. So we should be aware about such complications so that it can be helpful in managing such cases and also will help to prevent its occurrence.
Most of these complications take place intra-operatively because of pharmacological or mechanical insult to the delicate ocular structures and tissues.
Corneal Complications
Transparency of the cornea is absolutely essential to view the posterior segment during the vitreous surgery.
But sometimes during vitreous surgery corneal oedema occurs which occurs due to rise in intra-ocular pressure and sometimes due to corneal epithelial oedema. Both these conditions lead to decrease in corneal transparency because of there is difficulty in viewing the posterior segment.
When corneal epithelium is the cause of corneal oedema, it can be removed to improve the clarity. Lowering of the IOP further improves the clarity.
Persistence of corneal oedema in the post-operative period can be due to endothelial damage because of mechanical damage or prolonged exposure to irrigating fluids. This can be treated with hypertonic Sodium Chloride (5%) eye drops. The debrided epithelium usually heals in 48 to 72 hours of semipressure bandage except in diabetics where the healing is delayed. Topical phenylephrine should be avoided in debrided epithelium as it is toxic to the corneal endothelium.
Lens Complications
Damage to the crystalline lens can occur in the form of: -
-
Mechanical damage
-
Metabolic trauma
-
Damage from infusion line
-
Progressing of pre-existing cataract
There is also pharmacological and mechanical trauma to the lens intraoperatively.
Mechanical damage can occur during sclerotomy if it is too anterior, during introduction of infusion cannula or other intra-ocular instruments. This mechanical damage can be avoided by careful entry into the vitreous cavity.
The occurrence of mechanical injury to the lens often necessitates lens removal either with the cutter or framatome.
Pharmacological damage will occur if the infusion fluid is not supportive to the lens metabolism.
Cataract following Vitrectomy
Irrigating solution, contact of SF6 gas bubble with the lens can produce cataract. The incidence of cataract following vitrectomy is high in diabetics and a typical fer-like cataract develops.
Complications of Sclerotomies:
The site of sclerotomy should be within 3 to 4.5 mm from the limbus.
If it is too anterior it will damage the lens or ciliary body.
If it is too posterior it will damage the vireous base leading to retina dialysis or sometimes can lead to retina detachment.
There can be suprachoroidal infusion of the irrigating fluid if the infusion cannula is not properly placed in the vitreous cavity. This can happen in cases of hypotonus eyeball. This can be prevented with the use of a long infusion cannula.
There may occurrence incarceration of the peripheral retina at the sclerotomy site.
Bleeding from the sclerotomy site should be properly controlled.
Retinal complications:
1. Retina dialysis: -The causes of iatrogenic retinal dialysis are: -
-
Use of a blunt tipped instrument through a relatively narrow sclerotomy opening using pressure to push it in.
-
Use of a blunt vitreous cutter particularly of oscillatory type. This will cause wrapping of vitreous fibrils around the cutter which will lead to traction and eventually to retinal dialysis.
-
Because of the fluid from the infusion line if it has not been shut off during its insertion and removal. This can produce retinal as well as choroidal detachment.
2. Iatrogenic retinal holes/tears: -
These holes/tears will occur because of following reasons: -
-
Keeping the vitrectomy instrument very close to the retina during cutting the membrane with the vitreous scissors or while lifting the membrane with a pic or forceps.
-
Because of high suction which will lead to sucking the retina, during use of back flush or flute needle
-
Peripheral retina tears because of instrumental damage
-
Dialysis due to posterior location sclerotomy incision.
The production of such a tear is no longer considered a major complication as modern vitreous surgery often intentionally created retinotomies and retinectomies, whenever indicated, to relieve severe vitreo-retinal traction or to gain access to the subretinal space. The small tears can be managed with cryopexy or laser retinopexy.
Endophthalmitis
This is a potentially devastating complication. It is rare following pars plana surgery. It is managed in the same fashion as any postoperative endophthalmitis and reoperation may be considered for cases which worsen despite adequate medical therapy.
Postvitrectomy Glaucoma
There is usually a transient increase in the intraocular pressure in 50% of the cases following vitrectomy. They can in most of the cases medically managed. The common cause of this raised intraocular pressure in post-operative period are ghost cells blocking the trabecular meshwork, haemorrhage, phacolysis, steroid induced and neovascularisation of the angle structures.
If intraocular gas has been used, gas bubble expansion can cause glaucoma. Silicon oil pupillary block can also occur.
Recurrent vitreous haemorrhage:
This complication is common in diabetic eyes. The cause of recurrent vitreous haemorrhage may vary from trivial trauma to ongoing disease process.
Rubeosis Iridis
It is seen in commonly in aphakic eyes because of retinal hypoxia from any cause. It is usually seen between 3rd and 14th post-operative day.
References:
- Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: A pars plana approach. Trans Am Acad Ophthalmol Otolaryngol 1971;75:813-20
- Parel HM, Machemer R< Aumayr W: A new concept for vitreous surgery. Improvments in instrumentation and illumination. Am J Ophthalmol 77:6,1974
- Kwok AK, Tham CC, Lam DS, Li M, Chen JC. Modified sutureless sclerotomies in pars plana vitrectomy. Am J Ophthalmol 1999;127:731-3.
- Chen JC. Sutureless pars plana vitrectomy through self-sealing sclerotomies. Arch Ophthalmol 1996;114:1273-5.
- Kwok AK, Tham CC, Lam DS, Li M, Chen JC. Modified sutureless sclerotomies in pars plana vitrectomy. Am J Ophthalmol 1999;127:731-3.
- de Juan E Jr, Hickingbotham D. Refinements in microinstrumentation for vitreous surgery. Am J Ophthalmol 1990;109:218-20.
- Fujii GY, De Juan E Jr, Humayun MS, Pieramici DJ, Chang TS, Awh C,et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology 2002;109:1807-12;discussion 13.
- Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina 2005;25:208-11.
- Modern Ophthalmology. Edition 3;Vol 3 Page 1743


