Introduction
This chapter will discuss pediatric retinal vascular diseases like Persistent Fetal Vasculature, Familial Exudative Vitreo Retinopathy and Incontinentia Pigmenti
Persistent Fetal Vasculature (Persistent Hyperplastic Primary Vitreous)
Definition
Persistent hyperplastic primary vitreous (PHPV) is a congenital ocular disorder in which fetal vasculature does not regress after birth. It is unilateral approximately 90% of the time. No single gene has been identified. It can vary from very subtle with no visual disturbance to as severe as phthisis bulbi or secondary glaucoma. The prognosis can be good if early intervention is planned. Due to its wide spectrum of presentation, it has now been renamed as persistent fetal vasculature (PFV).1
Pathogenesis
The fetal vasculature is composed of two parts:
- Tunica vasculosa lentis. It is situated anteriorly encircling the lens. It has anterior and posterior divisions. Anterior division has additional attachments to the pupillary frill of the iris. Posterior division has additional attachments to the ciliary process and continues with the hyaloid artery posteriorly.
- Hyaloid artery. It is situated porteriorly behind the lens. It is also called primary vitreous. The hyaloid vessel extends from posterior surface of lens to the disc. The vasculature fills the vitreous cavity & has many attachments to the retinal surface
This new term of PFV emphasizes the importance of both anterior tunica vasculosa lentis and posterior persistent hyaloid system and also represents the spectrum of structural changes which can present within the eye.
Normal regression of embryonic vascular system2
During development blood flow to the eye is through hyaloid artery. At the 240-mm stage (seventh month) in the human, blood flow in the hyaloid artery ceases. Hyaloid vascular regression occurs in following manner:
Clinical spectrum of PFV
There is a spectrum of disorders resulting from persistence of the fetal vasculature.
- Mittendorf’s dot is a remnant of the former site of anastomosis of the anterior tunica vasculosa lentis and posterior hyaloid artery. It is usually inferonasal to the posterior pole of the lens and is not associated with any known visual dysfunction.
- Bergmeister's papilla is the occluded remnant of posterior portion of the hyaloid artery, associated with glial tissue. It appears as a gray, linear structure anterior to the optic disc. It also has no visual dysfunction.
- Vitreous cysts are generally benign lesions that are found in eyes with abnormal regression of the anterior or posterior hyaloid vascular system. It can occur in otherwise normal eyes or eyes with coexisting ocular disease, such as retinitis pigmentosa, retinochoroidal colobomas and uveitis. They are generally not symptomatic and thus do not require surgical intervention.
- PHPV (PFV): Although it is a non-progressive disorder, the tractional intraocular changes can occur later, most likely due to eye growth. The stalk also can cause traction on the posterior lens capsule leading to posterior lenticonus. Traction on the ciliary body can lead to hypotony. Traction on the retina, leads to tractional retinal detachment.
Classification
There are three types of PFV:
- Anterior PFV: It has predominant features of persistent anterior tunica vasculosa lentis without much or any posterior hyaloid component.
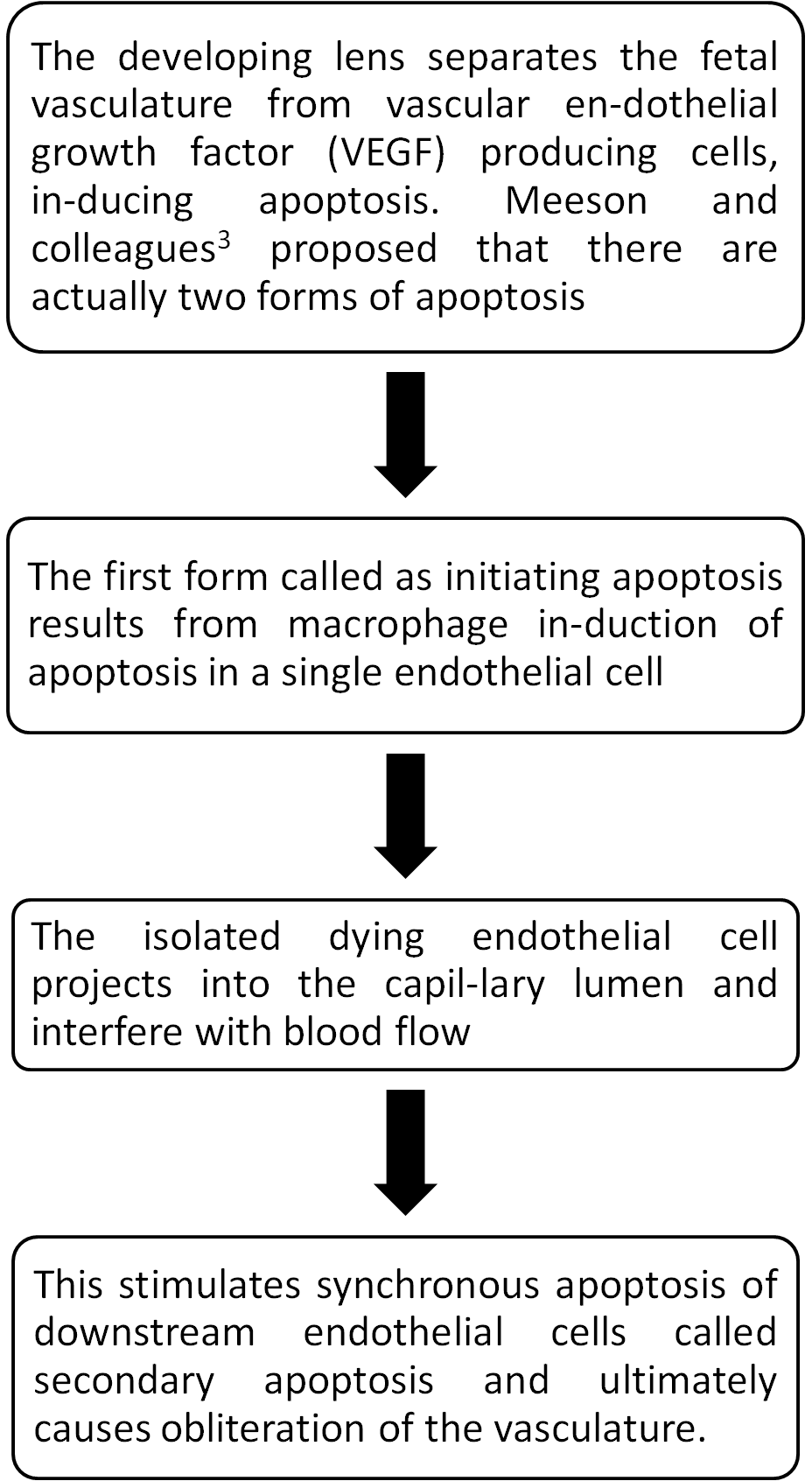
Clinical features (Figure 1A):
- Presentation age 1 – 2 weeks with leukocoria.
- Microphthalmos.
- Posterior lens opacity → cataract.
- Retrolental fibrovascular membrane.
- Shallow anterior chamber → glaucoma.
- Elongated ciliary process → hypotony.
- Stalk extending from posterior part of lens to optic disc may-not be present.
- Posterior PFV: It has predominant features of persistent posterior hyaloid artery without much or any anterior tunica vasculosa lentis.
Clinical features:
- Microphthalmos (may or may-not be present).
- Posterior lens opacity.
- Vitreous stalk (Figure 1B). The stalk can insert anteriorly on the lens either centrally, involving the visual axis or eccentrically (nasally), sparing the visual axis of the lens. If the stalk is eccentric, no change in visual acuity is noticed in the very young child, but strabismus can result. If there is visual axis opacity, the problem usually is discovered earlier. The child with a stalk eccentric to the visual axis often presents with strabismus later in life, at age 9 to 10 months.4
- Retinal fold.
- Tractional retinal detachment.
- Hypoplastic optic nerve & macula.
- Mixed PFV: When both the tunica vasculosa lentis and hyaloid system is present then it is the mixed type of PFV. It has a spectrum of presentations depending on the degree of involution of the hyaloid and tunica vasculosa lentis.
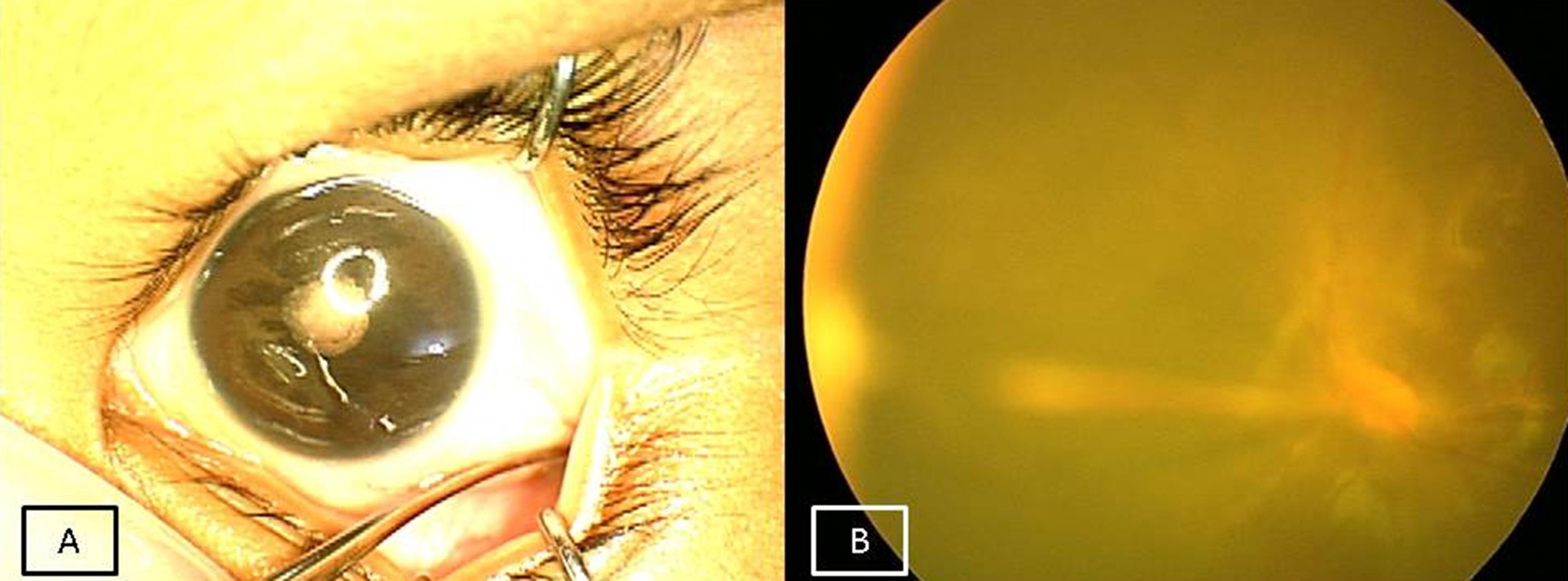
Figure 1A: Anterior PFV showing microphthalmos with cataract. 1B: Fundus picture of left eye showing stalk extending from optic disc to lens suggestive of posterior PFV.
Investigations
- B scan ultrasound:5 Echography usually shows a shorter than normal globe, although it may be normal in some patients. The lens is often thin and there may be irregularity of the posterior capsule. A retrolental membrane can sometimes be demonstrated. A vitreous band (persistent hyaloid vessel) may be seen extending from the posterior lens capsule to the optic disc. Very often this band is extremely thin and difficult to identify along its entire course (Figure 3). In other situations it can be extremely thick and easy to demonstrate. Sometimes a tractional retinal detachment can be seen posteriorly which can help differential it from a closed funnel retinal detachment.
- Ultra Biomicroscopy (UBM): Can show the fibrous membrane behind the lens. May require general anesthesia.
- Computed tomographic scanning and magnetic resonance imaging are rarely needed, mainly to rule out retinoblastoma in doubtful cases.
Treatment
- Observation: If there are subtle persistent fetal vasculature changes that do not affect the visual axis then the child is only observed and refractive correction with amblyopia management advised. On the other side of the spectrum, if the eye is severely microphthalmic with a closed funnel retinal detachment with subretinal opacities, then these eye also need to be only observed and proper cosmetic correction given
- Surgery:
- Anterior PFV is managed similar to congenital cataract where through scleral tunnel lens aspiration is done followed by primary posterior capsulotomy followed by removal of the fibrous membrane and anterior vitrectomy. Bleeders are managed by diathermy. Post operatively refraction and aggressive amblyopia management is required. Posterior chamber intraocular lens (PCIOL) can be placed in children more than 6 months of age in sulcus if capsular rim can be kept intact.
- Posterior PFV: Surgery not required if no traction on lens capsule, retina and clear visual axis. Lens sparing vitrectomy6 is the surgery of choice (however lensectomy is mostly needed due to the close proximity with the lens). Entry is through pars plicata (as pars plana is still not developed in infants). Thus entry is 1.5 mm from limbus instead of 4 mm (as in adults) to avoid the anterior insertion of the retina and the creation of itrogenic breaks. After core vitrectomy the stalk is cut. Intrinsic retinal vessels can be present in stalk. Stalk can be pushed from side to side before dividing to determine any retinal circulation alteration. If intrinsic vessels present then the stalk is usually cut anteriorly. The surgeon should be aware of this as great care is required to divide only stalk tissue, not retina or intrinsic retinal vessels which could lead to a retinal hole or bleeding, followed by a retinal ischemic event. At the end fluid air exchange can be done.
- Mixed PFV: Cataract aspiration is done as anterior PFV. This is followed by three port vitrectomy either through the limbal paracentesis using anterior chamber maintainer or through the pars plicata after making the ports 1 mm from limbus.
Care should be taken during surgical removal of fibrous membrane and peripheral vitrectomy as it can easily lead to itatrogenic retinal dialysis.
Postoperative Treatment
- Surgery alone does not constitute treatment
- Aggressive management of amblyopia by way of contact lenses, aphakic glasses or secondary PCIOL implantation is needed to have a good visual outcome
Visual prognosis
The severity of anterior segment appearance, globe size, the severity of lens involvement and retinal dysplasia often determines the eye's visual result. Retinal dysplasia can be divided into macroscopic and microscopic types.6 Macroscopic dysplasia is defined as changes easily visible with the operating microscope or indirect ophthalmoscope. Microscopic dysplasia occurs at the cellular or vascular level. Vascular dysplasia can be assessed by fluorescein angiography where a capillary-free zone is seen. Visual evoked potential testing may be useful in predicting retinal dysplasia.7 If a response is present, then it can be helpful in the decision whether to recommend surgery for retinal detachment. This is most helpful when the other eye is uninvolved, and the waveforms can be compared.
Differential Diagnosis
- Retinoblastoma
- Norrie disease: When bilateral PFV syndrome is present.
- Congenital cataract.
- Familial exudative vitreoretinopathy.
- Incontinentia pigmenti.
- Stage 5 Retinopathy of prematurity.
Other systemic associations have been reported with PFV, most often in association with central nervous system problems.8 Associations have also been reported with intrauterine herpes simplex virus infection,9 anterior and posterior colobomas or even cystic globes10 and tuberous sclerosis.11
Conclusion
- With modern vitreoretinal techniques, good visual results are possible. Smaller gauge 25 and even 27 G instruments can be used for vitrectomy.
- However good anatomical and visual outcome depends on
- Timing of Intervention
- Anatomy of Posterior Pole
- Amount of Anisometropia
- Amblyopia Management
Familial Exudative Vitreoretinopathy
Definition
Familial exudative vitreoretinoapthy (FEVR) is a retinal vascular disease in which the peripheral retinal vessels fail to grow into the peripheral retina, leaving areas of avascular retina. FEVR was first described by Criswick and Schepens in 1969.12 It is referred to as retinopathy of prematurity (ROP) in full-term infants because the clinical findings resembled those of ROP but were observed in full-term babies.
Unlike ROP which resolves once the infant attains maturity, FEVR is a lifelong vascularly active process with variable periods of quiescence. FEVR varies in presentation, course and inheritance from person to person. It is generally agreed that children who present with FEVR before the age of 3 years have a worse prognosis.
Genetics
The most common pattern of inheritance is autosomal dominant, although families with X-linked and autosomal recessive forms have been reported.13 The loci for two autosomal dominant forms (EVR1 and EVR3) and an X-linked form (EVR2) of FEVR have been mapped. EVR1 and EVR3 are both on chromosome 11 (11q13-q23 & 11pl3-pl2). Recently, in Indian patients, novel FZD4 mutations are also found.14
Clinical features
The clinical features of FEVR can vary from
- Peripheral avascular retina to
- Neovascularization on edge of avascular retina to
- Retinal exudation to
- Vitreous haemorrhage to
- Partial retinal detachment (RD) with or without macular folds (Figure 2A) to
- Leukocoria secondary to total RD.
RD can be tractional or exudative (most of the time combination of both). Atrophic holes are also often seen the avascular retina
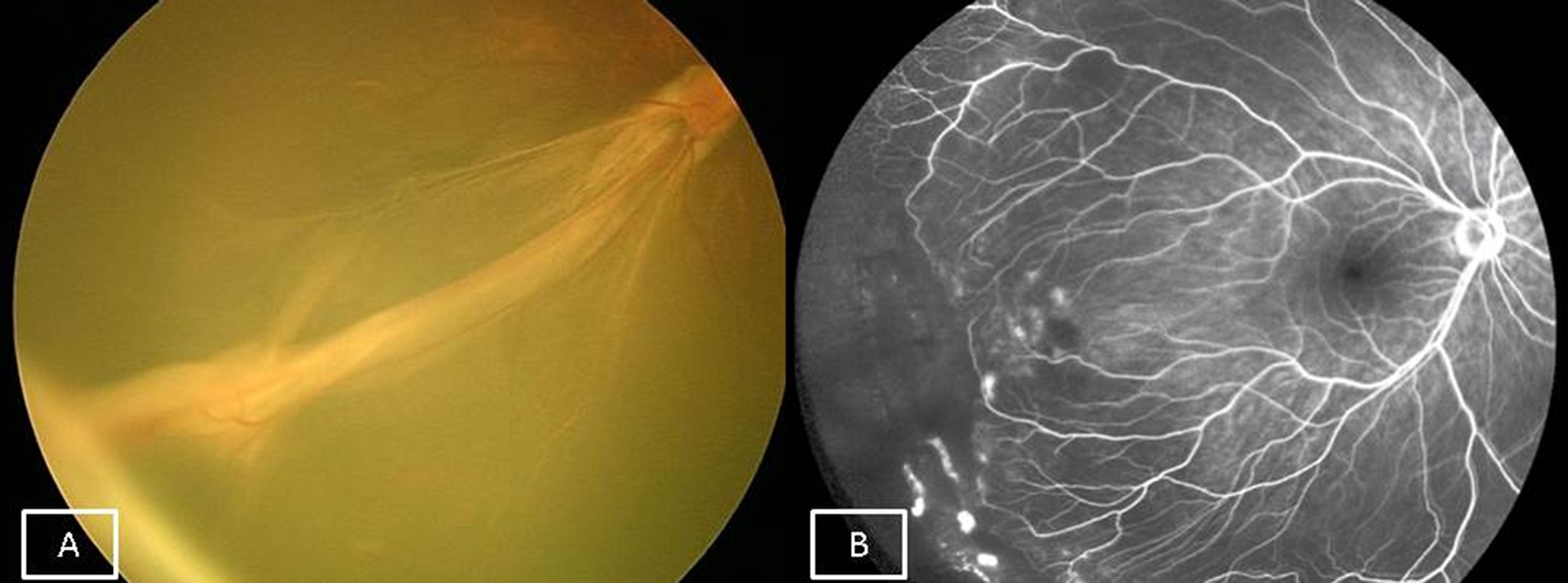
Figure 2A: Fundus picture of right eye showing tractional macular fold suggestive of Stage 4A FEVR. 2B: Wide field FFA of right eye showing peripheral avascular retina temporally and few leaks from new vessels suggestive of stage 2A FEVR.
Classification15
Stage 1: Avascular retinal periphery only.
Stage 2: Avascular retinal periphery with extra-retinal neovascularisation
A: without exudate.
B: with exudate.
Stage 3: Subtotal retinal detachment (RD) without involving the fovea
A: without exudate.
B: with exudate.
Stage 4: Subtotal RD involving the fovea
A: without exudate.
B: with exudate.
Stage 5: Total RD (open/closed funnel)
A: open funnel
B: closed funnel
(Note: Stages 2, 3 and 4 are subdivided into A or B, depending upon primarily tractional or exudative components, respectively)A thorough peripheral retinal examination should be recommended for family members as the disease may present in the mild form without any symptoms. Even though the disease is commonly bilateral (85%) it may be asymmetrical in its presentation. The diagnosis is often made by examining the lesser involved eye because the features may not be recognizable in the more involved eye due to end-stage disease.
Investigations
Fundus fluorescein angiography (FFA) is performed to make the correct diagnosis. It shows capillary non-perfusion areas in the periphery with or without leakage at the edge, depending on the presence of new vessels. As FEVR is mainly a disease of the periphery, wide-field angiography is preferred now (Figure 2B).
Treatment
Like many treatments for pediatric retinal diseases, early intervention often results in better visual results. Screening is particularly important in preverbal children. FEVR often has a progressive long-term course, with periods of waxing and waning, and requires lifelong screening, particularly with new symptoms such as reduced vision or floaters.
- Observation: Stage 1 FEVR with only peripheral avascular retina need to be only closely observed.
- Prophylactic barrage laser: Barrage laser is done to cases where atrophic retinal holes are seen in the avascular retina in the periphery, to prevent rhegmatogenous RD.16
- Laser photocoagulation: If any vascular proliferation is seen at the juncture of the avascular and vascularized retina, then laser of the avascular peripheral retina is needed to decrease the stimulus for neovascularization.17 The entire avascular retina is ablated with near confluent burns, like ROP.
- Scleral buckle: Maybe helpful in sone detachments with peripheral traction.
- Vitrectomy with silicon oil: It has a considerable role in the management of advanced FEVR as most present with extensive vitreous membranes and tractional detachment. Encircling band is usually recommended as it is very difficult to induce posterior vitreous detachment till periphery and many times a significant amount of peripheral vitreous is felt behind. Silicone oil helps to stabilize the retina by providing long-term tamponade. 5000 centistokes silicon oil is preferred as it can be left for a longer duration.
- Enzymatic vitrectomy: Plasmin assisted vitrectomy has also been tried in selective pediatric diseases and has a potential to be used in FEVR vitrectomy also, in the future.18
- Anti-vascular endothelial growth factor (VEGF) injections: Anti-VEGF injections like bevacizumab can be tried to reduce the exudation,19 however additional laser is required to the avascular retina. Caution should be used while injecting in cases with fibrous proliferation, as anti-VEGF injections are known to contract them and worsen the detachments.20
Differential diagnosis
- ROP (will have history of prematurity, oxygen at birth)
- Norrie’s disease (X linked recessive, so mainly males only affected. Presence of mental retardation and hearing loss).
- Persistent fetal vasculature (usually unilateral).
- Incontinentia pigmenti (X-linked dominant, so mainly females only affected and history of skin lesions over extremities at birth).
- Retinoblastoma (Obvious mass with calcification seen on ultrasonography).
Incontinentia Pigmenti
Definition
Incontinentia Pigmenti is a congenital multi-system disorder which in the eye presents as a vasoproliferative disorder of the retina that can cause total retinal detachment and blindness.21 Other systems that get involved are skin, central nervous system (CNS), teeth and hair.
Genetics
It is an X-linked dominant disease and hence only females get affected. The gene identified is the NEMO gene present on the long arm of X chromosome. Mutations in the NEMO gene cause this disease.
Clinical features
Ocular
The commonest ocular features are peripheral avascular retina (Figure 3A) with abnormal vessel proliferation which can lead to retinal folds, total retinal detachment and blindness. Other rarer ocular findings are strabismus, congenital cataract and microphthalmos.
Skin
Skin lesions appear at birth over all four limbs (Figure 3B) and are characterized by four stages (vesicular, verrucous, hyperpigmented and hypopigmented). These lesions are innocuous and resolve over a period of time. Skin biopsy is diagnostic.
CNS
CNS findings are usually due to ischemia and can lead to stroke, seizures, periventricular leokomalacia and encephalopathy. Neuro imaging and symptomatic treatment is required bt a neurologist.
Dental
Dental abnormalities like missing, small or abnormally shaped teeth are seen over a long run, which have to be managed by a dentist.
Hair
Alopecia is common.
Figure 3A: Fundus picture of left eye of a child with incontinentia pigmenti showing posterior pole haemorrhages with temporal peripheral avascular retina. 3B: Typical skin lesions of incontinentia pigmenti affecting the extremities.
Investigations
FFA should be done in cases of suspected new vessels to confirm the diagnosis. Sometimes macular ischemia is seen which can be picked up only by FFA.
Treatment
- Screening: A through ocular screening with a detailed fundus examination should be done for all cases with suspected incontinentia pigmentii.
- Laser photocoagulation: ROP style near confluent laser photocoagulation in done to the entire avascular retina.
- Vitrectomy: Can be performed in cases with progressive retinal detachment. However the prognosis is poor.
- Anti-VEGF: Recently intravitreal bevacizumab22 has been shown to be effective in regressing the active new vessels.
References
- Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol 1997;124:587-626.
- Sebag J, Nguven N: Embryology of the posterior segment and developmental disorders. B. Vitreous embryology and vitreo-retinal developmental disorders. In: Hartnett ME, editor. Pediatric Retina. Philadelphia. Lippincott Willaims & Wilkins, 2005. pp 13-28.
- Meeson A, Palmer M, Calfon M et al. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development 1996;122:3929.
- Shaikh S, Trese MT. Lens-sparing vitrectomy in predominantly posterior persistent fetal vasculature syndrome in eyes with nonaxial lens opacification. Retina 2003;23:330-4.
- Byrne SF, Green RL. Intraocular tumors. In: Byrne SF, Green RL, editors. Ultrasound of the eye and orbit. Second edition. St. Louis. Mosby, 2002. pp 115-90.
- Trese MT, Capone A Jr. Diagnosis and Management of Persistent Fetal Vasculature Syndrome. In: Hartnett ME, editor. Pediatric Retina second edition. Philadelphia. Lippincott Williams & Wilkins, 2014. pp 626-32.
- Dass AB, Trese MT. Surgical results of persistent hyperplastic primary vitreous. Ophthalmology 1999; 1 06:280-4.
- Marshman WE, Jan JE, Lyons CJ. Neurologic abnormalities associated with persistent hyperplastic primary vitreous. Can J Ophthalmol 1999;34:17-22.
- Corey RP, Flynn JT. Maternal intrauterine herpes simplex virus infection leading to persistent fetal vasculature. Arch Ophthalmol 2000;118:837-40.
- Pasquale LR, Romayananda N, Kubacki J, Johnson MH, Chan GHl. Congenital cystic eye with multiple ocular and intracranial anomalies. Arch Ophthalmol 1991;109:985-7.
- Milot J, Michaud J, Lemieux N, Allaire G, Gagnon MM. Persistent hyperplastic ptimary vitreous with retinal tumor in tuberous sclerosis: report of a case including tumoral immunohistochemistry and cytogenetic analyses. Ophthalmology 1999; 106:630-4.
- Criswick VG, Schepens CL. Familial exudative vitreoretinopa¬thy. Am J Ophthalmol l969;68:578- 594.
- Plager DA, Orgel IK, Ellis FD, et al. X-linked recessive familial exudative vitreoretinopathy. Am J Ophthalmol 1992;114:145-14.
- Nallathambi J, Shukla D, Rajendran A, Namperumalsamy P, Muthulakshmi R, Sundaresan P. Identification of novel FZD4 mutations in Indian patients with familial exudative vitreoretinopathy. Mol Vis 2006;12:1086-92.
- Pendergast SD, Trese MT. Familial exudative virtreoretinopathy Results of surgical management. Ophthalmology 1998;105: 1015-1023.
- Shukla D, Singh J, Sudheer G, Soman M, John RK, Ramasamy K, Perumalsamy N. Familial exudative vitreoretinopathy (FEVR). Clinical profile and management. Indian J Ophthalmol 2003;51:323-8.
- Drenser KA, Trese MT, Capone A Jr. Familial exudative virtreoretinopathy (FEVR). In: Hartnett ME, editor. Pediatric Retina second edition. Philadelphia. Lippincott Williams & Wilkins, 2014. pp 345-9.
- Lee YS, Wang NK, Chen YP, Chen KJ, Hwang YS, Lai CC, Wu WC. Plasmin Enzyme-Assisted Vitrectomy in Pediatric Patients with Vitreoretinal Diseases. Ophthalmic Res 2016;56:193-201.
- Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Rapid regression of retinal hemorrhage and neovascularization in a case of familial exudative vitreoretinopathy treated with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol 2008;246:1787-9.
- Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2008;246:1061-3.
- Baranano DE, Goldberg MF. Incontinentia pigmenti. In: Hartnett ME, editor. Pediatric Retina second edition. Philadelphia. Lippincott Williams & Wilkins, 2014. pp 354-60.
- Shah PK, Bachu S, Narendran V, Kalpana N, David J, Srinivas CR. Intravitreal bevacizumab for incontinentia pigmenti. J Pediatr Ophthalmol Strabismus 2013 Oct 29;50 Online:e52-4.


