Glaucoma, by definition, is chronic, progressive optic neuropathy.[1-3] Structural damage to the optic nerve head (ONH) is a characteristic feature of glaucoma and precedes visual field loss in most cases. Para-papillary changes may also be observed in eyes with glaucoma. The mere presence of raised intraocular pressure is not glaucoma. Hence, the optic disc evaluation is the first and most important step in diagnosing glaucoma. [4, 5]
Subjectivity and overlap in appearance of the normal and glaucomatous optic disc make ONH evaluation challenging. Subtle changes may also be missed easily. Adequate training and experience are required to determine minor abnormalities in the ONH accurately. [6]
Examination of the optic disc
Direct ophthalmoscopy, Slit lamp biomicroscopy with a handheld +66, +78 or +90 dioptre aspheric convex lens or Hruby lens, or a contact lens (Goldmann lens) or Indirect ophthalmoscopy may be done to examine the ONH. The pupil should be dilated prior to the examination. A comparison between the various methods of examination is given below in Table 1.
Clinical examination is supplemented by stereophotography of the ONH. It serves as an objective record for documentation of current clinical findings, tool for comparison in the future and sometimes even captures details that are missed on clinical examination such as
Retinal Nerve Fibre Layer (RNFL) defects and splinter haemorrhages.[7]In the Ocular Hypertension Treatment Study (OHTS), 84% of haemorrhages on the ONH were detected on ONH photographs but were missed on clinical examination.[8]
|
|
Pros |
Cons |
|
Direct Ophthalmoscopy |
Portable, inexpensive, Magnified view |
Monocular, non-stereoscopic view |
|
Slit lamp biomicroscopy with handheld lens |
Stereopsis, Magnification, Comfortable |
|
|
Slit lamp biomicroscopy with contact lens |
Non-inverted image |
Uncomfortable, requirement of coupling fluid, longer duration |
|
Indirect Ophthalmoscopy |
Portable, stereoscopic view, Easier in children, uncooperative patients and in severe lens opacities |
Low magnification |
The normal optic disc
The optic disc appears as a vertically oval structure with average dimensions of 1.92 ± 0.29 (0.96-2.91) mm vertically, 1.76 ± 0.31(0.91-2.61) mm horizontally and 2.69 ± 0.70 (0.805.54) mm2 in surface area, in the posterior pole where the axons of the retinal ganglion cells and blood vessels converge to exit and enter, respectively, the scleral canal. The size of the optic nerve head is determined by the size of the scleral canal. Hence, high myopes typically have large scleral canals and large discs, while high hyperopes have small scleral canals and small discs. Optic disc dimensions show considerable inter-individual variability. In Indian eyes, the optic disc has been shown to have a surface area varying between 2.25-3.37 mm2.[6, 9, 10] The optic disc margin is seen as a white, circular band at the edge of the disc, which marks the edge of the scleral canal.[11]
The optic cup appears as a horizontally oval pale depression, centrally located on the ONH and is devoid of neural and glial tissue, thereby exposing the lamina cribrosa. [12]Fig 1 shows optic discs in both eyes of a patient with symmetrical and normal cupping.
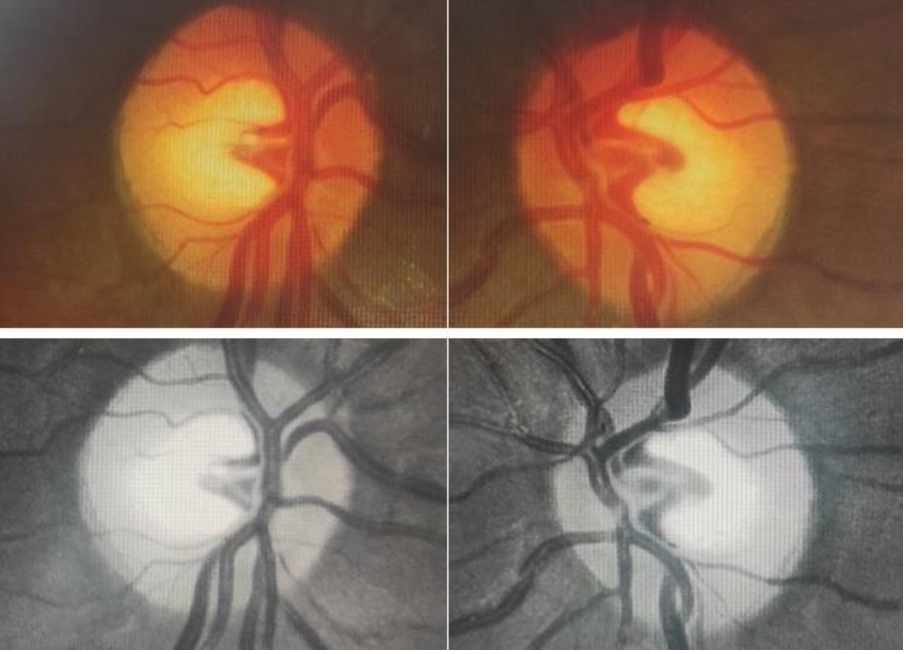
Physiologically, larger discs have larger cups and smaller discs have smaller cups. The boundaries of the cup are best determined by the double bending of the circumlinear blood vessels at the cup edge, rather than merely by the colour of the structures. The healthy neuroretinal rim has a pinkish-orange colour and typically follows the ‘ISNT rule’, whereby the inferior rim is thickest (18% thicker than the superior rim),[13] followed by the superior, nasal and temporal rim in the same order. This is a result of the peculiar arrangement of the RNFL (axons of retinal ganglion cells). [10, 12]Axons from the larger temporal retina are organized into superior and inferior arcuate fibres by the horizontal raphe and enter the disc at the superior and inferior poles, respectively, while the fibres from the smaller area of macula take a direct course via the papillomacular bundle and enter the ONH temporally. Fibres from the nasal retina take a straight course entering the ONH nasally.[14]
The five ‘R’s for assessment of the optic disc in glaucoma
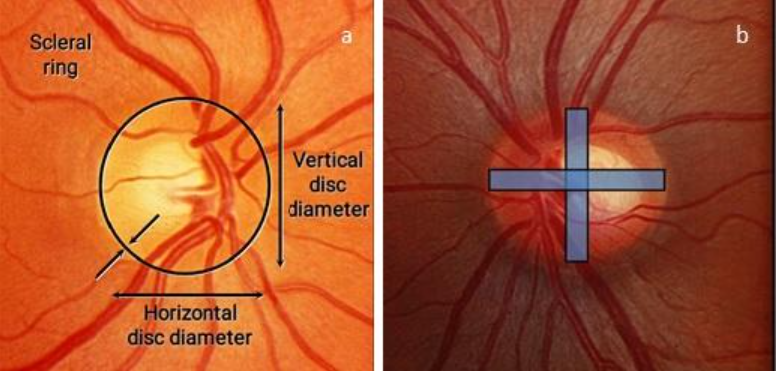
1. Scleral Ring:
Determine the size and shape of the optic disc by comparing it with the size of the slit beam. The correction factor to be applied for the Volk +60D, +78D, and +90D lenses are ×1.0, ×1.1 and ×1.3, respectively. [15, 16]The average vertical diameter of the optic disc is 1.8mm and the average horizontal diameter is 1.7mm. Optic discs <1.5mm in diameter vertically are considered small, and those >2.2mm in diameter vertically are considered large. The scleral ring should not be included as a part of the optic disc area as this may falsely increase the size of the neuroretinal rim and decrease the cup to disc ratio. Fig 2 shows the process of determination of the scleral ring and optic disc size.
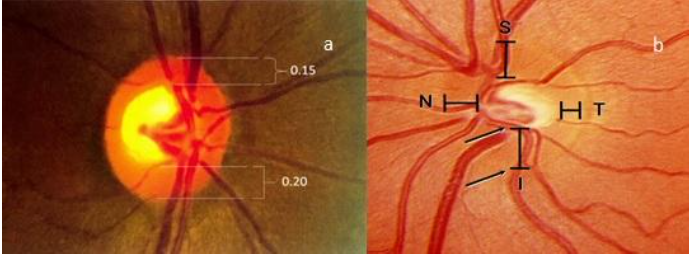
2. NeuroretinalRim:
Determine size of the neuroretinal rim by double-bending of the vessels and look for pallor and the ‘ISNT’ rule. The cup to disc ratio is estimated by assigning a value of 1 to the disc diameter in any meridian and proportionally estimating the cup size in the same meridian. An alternate method is to calculate the neuroretinal rim width in each sector in decimals relative to the optic disc size and then subtract from 1. Fig 3 shows the measurement of neuroretinal rim thickness and the ‘ISNT’ rule.
3. Retinal nerve fibre layer:
Look for the brightness of striations and visibility of parapapillary retinal vessels.
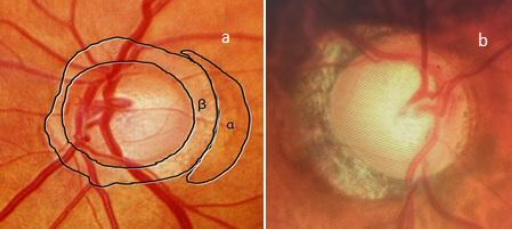
4. PeripapillaryRegion:
Look for atrophy in the alpha and beta zones. Hypo and hyperpigmented areas in the alpha zone may be present in normal as well as glaucomatous eyes. However, in the beta zone, atrophy of the retinal pigment epithelium and choriocapillaris are more common in glaucomatous eyes.[17]Fig 4 shows an examination of the peripapillary region.
5. Retinal and optic disc haemorrhages:
Careful examination of the disc should be done for splinter haemorrhages.
Commonly seen ONH changes in glaucoma
1. Increase in the cup-to-disc ratio
A cup to disc ratio of ≥0.6 should be viewed with suspicion of glaucoma. In established glaucoma, an increase in the cup to disc ratio signifies disease progression. A deep, excavated cup may also indicate the progression of glaucoma.
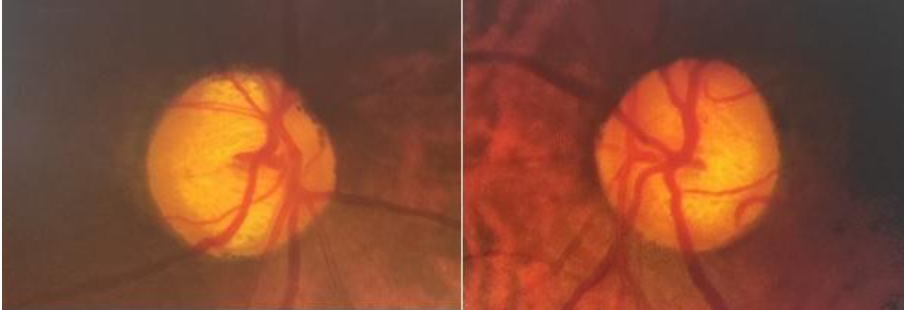
2. Asymmetry of the cup to disc ratio
An asymmetry of ≥0.2 between the cup to disc ratio of both eyes in the presence of similar disc sizes should prompt further evaluation to rule out glaucoma. However, asymmetry of cupping may also be seen in congenital anomalies like optic disc hypoplasia, morning glory disc, disc coloboma and congenital optic disc pit, as well as in anisometropia. Fig 5 demonstrates increased cupping and asymmetry of cupping between the eyes.
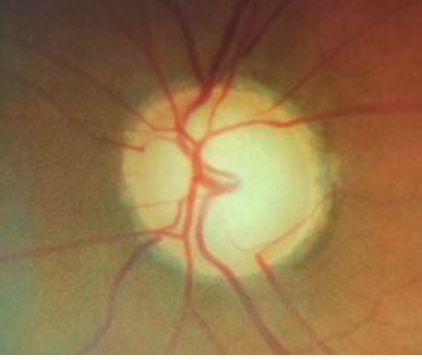
3. Loss of ISNT rule, diffuse neuroretinal rim thinning or loss and localised neuroretinal rim loss/notching
Loss of ‘ISNT’ rule is an early sign of glaucoma. The neuroretinal rim is the intrapapillary equivalent of RNFL. Loss of retinal ganglion cell axons in glaucoma is reflected as thinning or notching of the neuroretinal rim, as demonstrated in Fig 6.
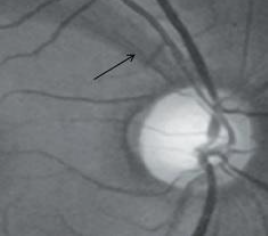
4. Nasalization of vessels
Circumlinear vessels are seen to enter and leave the eye along the nasal margin of the cup. However, this finding is not specific to glaucoma and may be seen in all large cups, as shown in Fig 7.
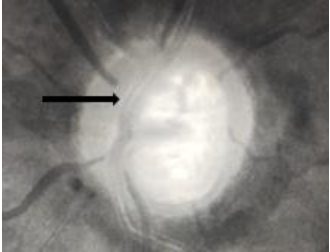
5. RNFL defect
Examination of the RNFL is done in a dilated pupil with a noncontact lens in red free illumination on the slit lamp. They are seen as bright striations and are most clearly seen in the inferior and superior temporal sectors than nasally. The RNFL becomes less clearly visible with increasing age. Also, the vessels embedded within the RNFL are better seen in eyes with RNFL defects. These defects may be wedge-shaped localised defects (≤60˚ on the optic disc margin) or generalized. However, they aren’t specific for glaucoma. RNFL defects are most common in normal-tension glaucoma, followed by primary open-angle glaucoma and then secondary open-angle glaucoma.[11]RNFL defects may succeed in disc haemorrhages after their resolution in the same region. They may be seen in areas of neuroretinal rim thinning or notching.[18]Fig 8 shows an RNFL defect.
6. Bayonetting
Circumlinear vessels pass under the overhanging edge of the cup and bend sharply by ≥90˚ at the cup margin. This is associated with neuroretinal rim thinning. Bean potting or shelving is a similar appearance of disappearance and re-appearance of the vessel under the margin of the excavated neuroretinal rim.[19]Fig 9 demonstrates bayonetting.
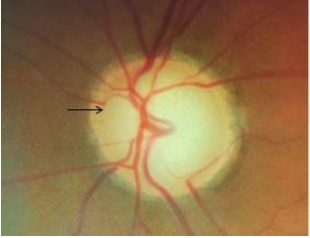
7. Baring of circumlinear vessels
Circumlinear vessels normally rest on the neuroretinal rim within the optic disc. Baring is described as the unsupported/suspended/hanging in mid-air appearance of the vessels over the optic cup due to loss of the adjacent neuroretinal rim (Fig 10). It is not pathognomonic of glaucoma.[19]
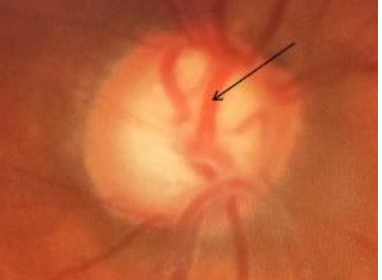
8. Saucerization
Diffuse, shallow cupping extending to the disc margin, as shown in Fig 11, is an early sign of glaucoma. The neuroretinal rim appears as a slope in these eyes.
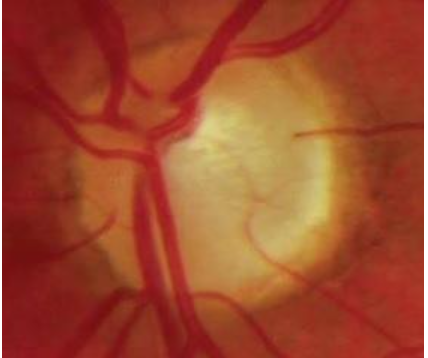
9. Disc haemorrhage
Feathery-edged, splinter or flame-shaped haemorrhages due to ischaemic microinfarction on the disc with radial orientation perpendicular to the disc margin are seen in glaucoma, as seen in Fig 12. These haemorrhages lie within the retinal nerve fibre layer and prelaminar disc and may be seen within one disc diameter of the optic disc. [11]However, other causes of haemorrhage should be ruled out. 1.9-16.9% of patients with glaucoma have optic disc haemorrhage.[20] The association is characteristically with normal tension glaucoma (5-9%, due to higher transmural pressure) than with primary open-angle glaucoma (4.3%). They may occasionally be bilateral. Disc haemorrhages are transient and last around 12.8±8.1 weeks. Disc haemorrhages signify progressive damage and is hence a poor prognostic sign.[19]
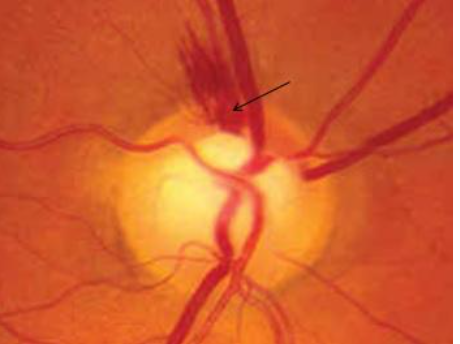
10. Thinning of arterioles
Focal constriction of vessels (Fig 13) seen close to the ONH is a sign of disease progression. The narrowing occurs as a response to decreased demand from the atrophic retina/ONH.[19]
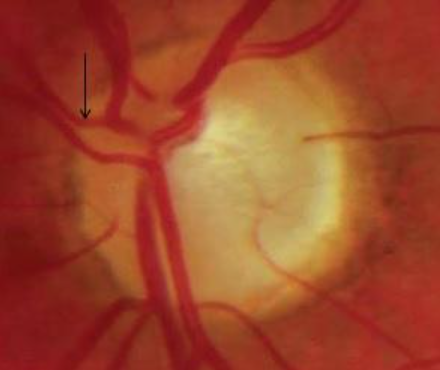
11. Laminar dot/slit sign
Visibility of the lamina cribrosa pores is more common in glaucomatous eyes (Fig 14). A rise in intraocular pressure in glaucomatous eyes causes posterior displacement of the lamina cribrosa, causing deformation of the pores and compression of the nerve fibres and blood vessels passing through them.Occasionally, the pores may be deformed into a slit shape in these eyes. The superior and inferior poles of the lamina cribrosa have less structural support and have the largest pores. Hence, the greater damage of the retinal ganglion cell axons at these poles. [19]
Conclusion
Examination of the ONH is an important step in the diagnosis of glaucoma and monitoring treatment. However, early ONH changes are easily missed and normal optic discs are determined abnormal in the learning years. While optical coherence tomography(OCT) and OCT angiography have made the detection of minor abnormalities easier, clinical examination remains the first and most vital diagnostic tool in the management of patients with glaucoma.
References
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974; 13(10): 771-83.
- Quigley HA, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol. 1976;15(8): 606-16.
- Quigley HA,Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981; 99(4): 635-49.
- Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991; 109(1): 77-83.
- Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999; 40(10): 2242-50.
- Reis ASC, Toren A, Nicolela MT. Clinical optic disc evaluation in glaucoma. EurOphth. 2012; 6(2): 92-7.
- Stone RA, Ying GS, Pearson DJ, et al. Utility of digital stereo images for optic disc evaluation. Invest Ophthalmol Vis Sci. 2010; 51(11): 5667-74.
- Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc haemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006; 113(12): 2137-43.
- Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988; 29(7): 1151-8.
- Harizman N, Oliveira C, Chiang A, et al. The ISNT rule and differentiation of normal from glaucomatous eyes. Arch Ophthalmol. 2006; 124(11): 1579-83.
- Gandhi M, Dubey S. Evaluation of the optic nerve head in glaucoma. JCurrGlaucoma Pract. 2013; 7(3): 106-14.
- Garway- Heath DF, Ruben ST, Viswanathan A, Hitchings RA. Vertical cup/disc ratio in relation to optic disc size: its value in the assessment of the glaucoma suspect. Br J Ophthalmol. 1998; 82(10): 1118-24.
- Arvind H, George R, Raju P, Ve RS, Mani B, Kannan P, et al. Neural rim characteristics of healthy South Indians: the Chennai Glaucoma Study. Invest Ophthalmol Vis Sci. 2008; 49(8): 3457-64.
- Hoyt WF, Frisen L, Newman NM. Fundoscopy of nerve fibre layer defects in glaucoma. Invest Ophthalmol. 1973; 12(11): 814-29.
- Ansari-Shahrezaei S, Maar N, Biowski R, Stur M. Biomicroscopic measurement of the optic disc with a high-power positive lens. Invest Ophthalmol Vis Sci. 2001; 42(1): 153-7.
- Garway-Heath DF, Rudnicka AR, Lowe T, Foster PJ, Fitzke FW, Hitchings RA. Measurement of optic disc size: equivalence of methods to correct for ocular magnification. Br J Ophthalmol. 1998; 82(6): 643-9.
- Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillarychorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989; 30(5): 908-18.
- Airaksinen PJ, Mustonen E, Alanko HI. Optic disc haemorrhages precede retinal nerve fibre layer defects in ocular hypertension. ActaOphthalmol. 1981; 59(5):627-41.
- Turgut B. Pearls for correct assessment of optic disc at glaucoma diagnosis. US Ophthalmic Rev. 2017; 10(2): 104-10.
- Healey PR, Mitchell P, Smith W, Wang JJ. Optic disc haemorrhages in a population with and without signs of glaucoma. Ophthalmology. 1988; 105(2): 216-23.

