Introduction:
Toxoplasmosis is the most common cause of posterior uveitis in the world, accounting for over 80% of the cases in some regions[1-5]. For many years, ocular toxoplasmosis was considered to be the result of the recurrence of the congenital form of the disease [6]. However, it is recently believed that acquired infections might be a more important cause of ocular diseases than congenital ones[7-9]. Acquired infections occur secondary to ingestion of uncooked and infected meat, contaminated vegetables, or water.
Epidemiology:
The Toxoplasma Gondii infection is one of the most common zoonoses in the world. A large percentage of the population has chronic asymptomatic disease[10]. The prevalence varies extensively in different regions, depending on socioeconomic, geographic, and climatic factors. A high prevalence is found in tropical areas close to sea level, and a lower prevalence is found in arid regions, cold climates, and at high altitudes. In addition, the habit of eating raw meat and the presence of the domestic cat greatly increase the incidence of Toxoplasma disease[11]
Pathogenesis:
T. gondii exists in 3 forms, trophozoites, the proliferating invasive form responsible for acute infection, bradyzoites, which is the encysted form found in tissue cysts and the oocysts produced only in cats and excreted in their feces during the sexual phase. The tachyzoite encysts at the sign of stress such as the host immune response or the presence of antibiotics[12]. These cysts are very resistant and can remain dormant in the host for years.
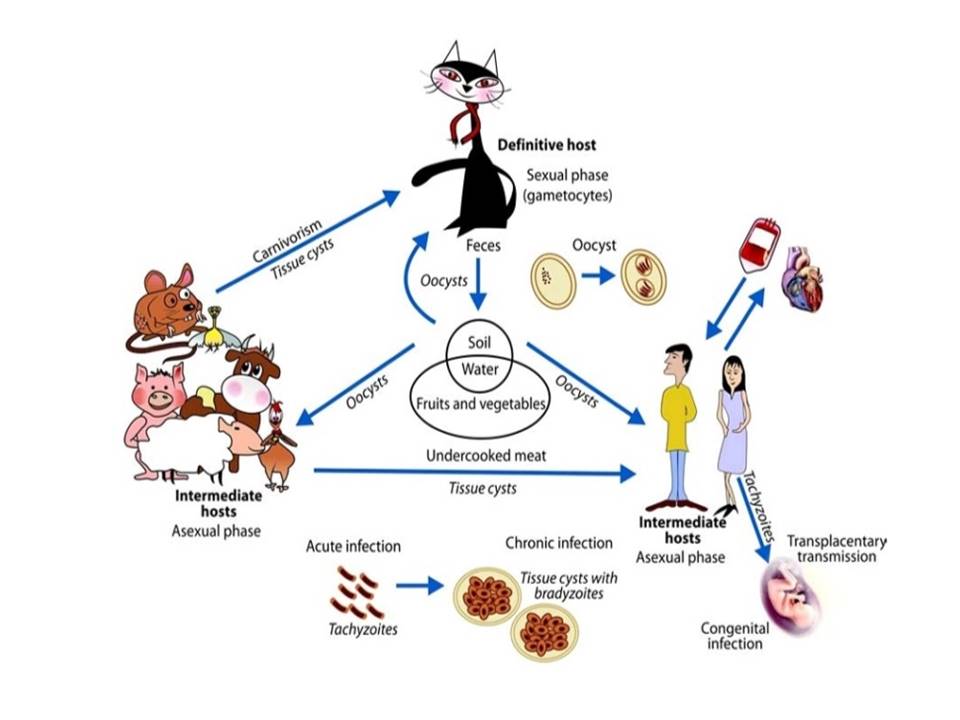
The life cycle of T. gondii is composed of two distinct phases: the asexual phase, which occurs in all hosts, and the sexual phase which occurs in the intestinal epithelium of the definitive host.
The majority of acute toxoplasmosis in immunocompetent hosts are subclinical and they yield a focus of inflammation after reaching the retina. The lesion is progressed to retinitis and involves the choroid secondarily. The cyst remains inactive in the scar or in the nearby retina for a long time and the retinitis may get reactivated when the cyst ruptures[14]. Hence the reactivation of retinitis develops at the border of old scars because of the rupture of tissue cysts located within old lesions. Sometimes, however, new lesions are found at locations distant from old scars.
Mode of transmission
The main mode of infection is by ingestion of oocysts from the cat feces present in soil and sandboxes. Oocysts attached to fruits and vegetables and oocysts in water might be a route of infection. However, ingestion of tissue cysts in raw or undercooked meat from several intermediate animals may be the main cause of infection in some countries. In addition, evidences show that contaminated drinking water could be a principal route of endemic infections with T. gondii[15,16]. An outbreak of acute active toxoplasmosis occurred in Coimbatore ,India in the year 2006 where a majority of cases were found to be located in the areas supplied by one water reservoir and the prima facie evidence suggested water to be the probable cause[17].
Transplacental transmission may occur if a woman is initially infected just before or during pregnancy. Postnatal infection is now thought to be a more common cause of ocular toxoplasmosis [7,18].
Clinical manifestations:
Congenital toxoplasmosis:
The incidence of ocular infection in congenital toxoplasmosis is 80%, most of which are subclinical and bilateral. The incidence of acquiring toxoplasmosis during pregnancy is only 0.2% to 1%.Among women who first contract toxoplasmosis during pregnancy, the chance of transplacental infection of the fetus is about 40%[19]. Infection occurring in the first trimester results in spontaneous abortion or birth of an infant with severe disease. Vertical transmission is most frequent during the third trimester when the fetus is exposed to maternal blood. Fortunately, third-trimester infection usually results in a subclinical form of the disease.[20,21].
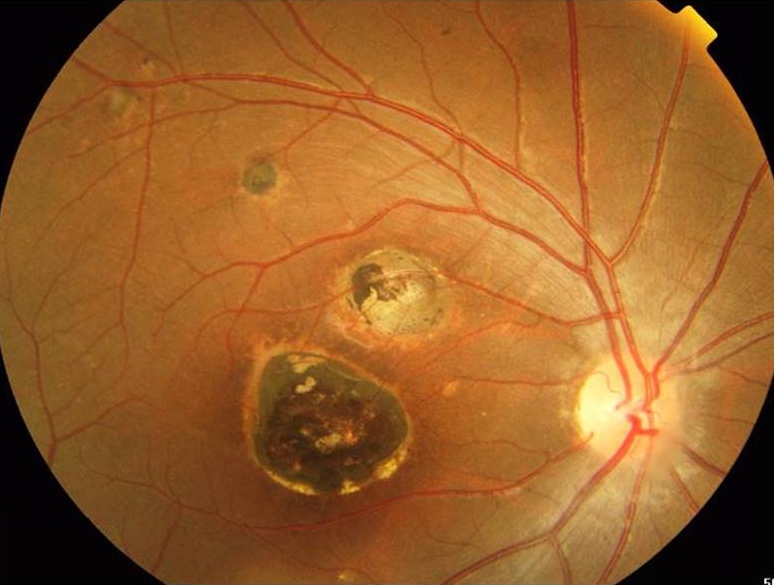
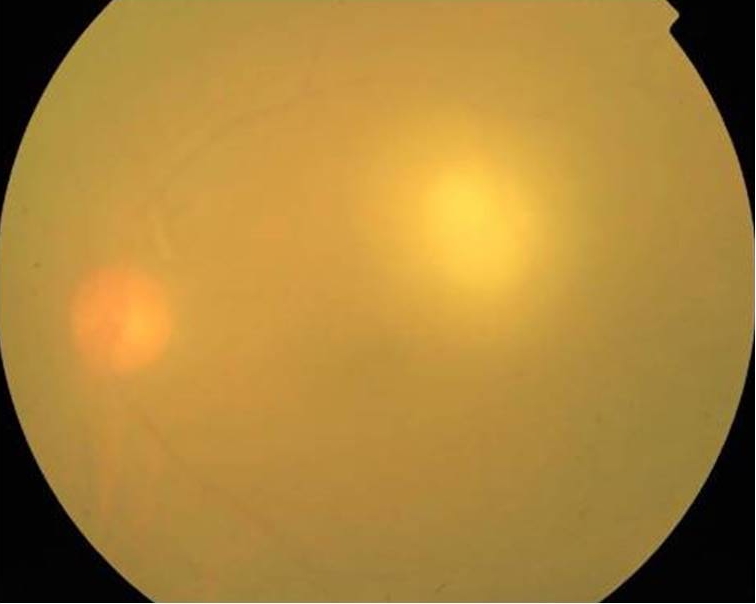
Congenital toxoplasmosis is bilateral and mostly has macular involvement (Fig2). Other clinical manifestations are intracranial calcification (32%), hydrocephaly or microcephaly (26%), and seizures. There is a predilection for the posterior pole because of the end-artery anatomy of the fetal macular circulation. The classic triad of congenital toxoplasmosis is retinochoroiditis, intracranial calcification, and hydrocephalus [22]. Children with mild infection present later in life with complaints of decreased visual acuity, strabismus, nystagmus, microphthalmia, optic atrophy and pthisisbulbi.
Acquired Toxoplasmosis
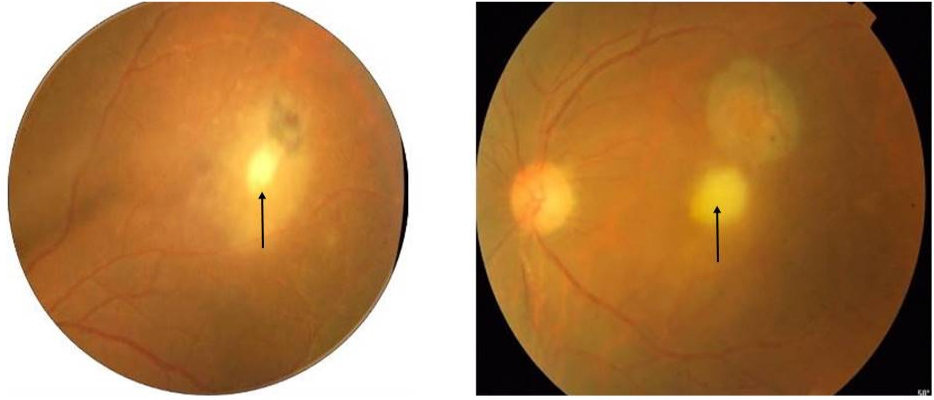
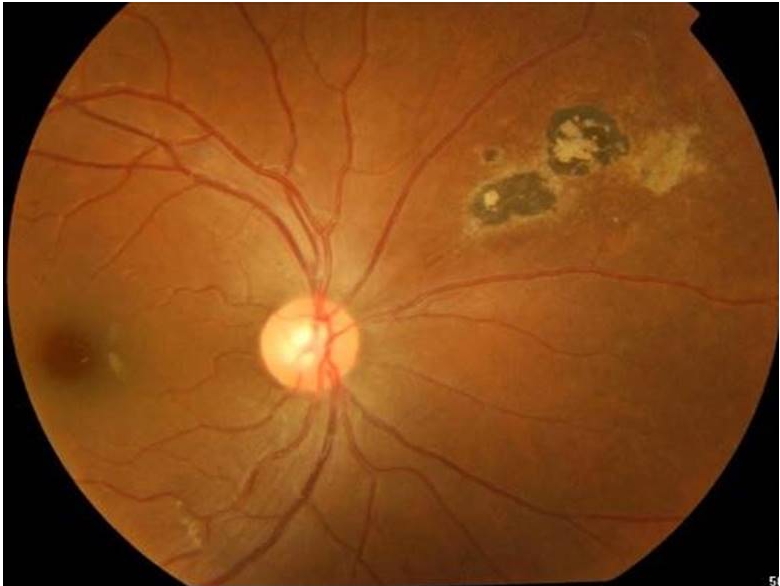
Typical toxoplasma lesions appear as white fluffy necrotizing retinitis adjacent to an old retinochoroidal scar and are called satellite lesions (fig3). Retinitis may also recur in distant sites away from the primary lesion or in the fellow eye and can be single or multiple[23]. The toxoplasma retinitis can progress involving the full-thickness retina, adjacent choroid, vitreous, and even sclera. The lesions resolve from centre to periphery and often leaves an atropic retinochoroidal scar.
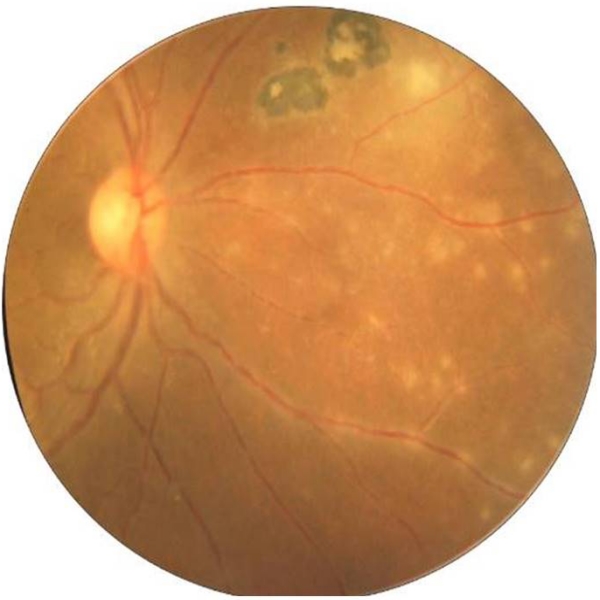
Patients who present with newly acquired ocular toxoplasmosis usually have unilateral, solitary, active lesions without evidence of previous retinochoroidal scarring. Immunocompetent patients may present with marked vitritis which is nearly present in all cases. On ophthalmoscopy examination, the active retinitis lesion appears as a bright white reflex through intense vitritis giving it a classic “headlight in the fog” appearance[24] (fig 4).
Healed lesions typically have well-defined borders with central retinochoroidal atrophy and peripheral pigment epithelial hyperplasia (Fig5). Healing lesions may be complicated by proliferative vitreoretinopathy, retinal gliosis, vascular shunts, and choroidal neovascular membranes. Traction bands linking an old scar to the optic disc (Franceschetti's syndrome) or to a neighboring scar are also common[23]
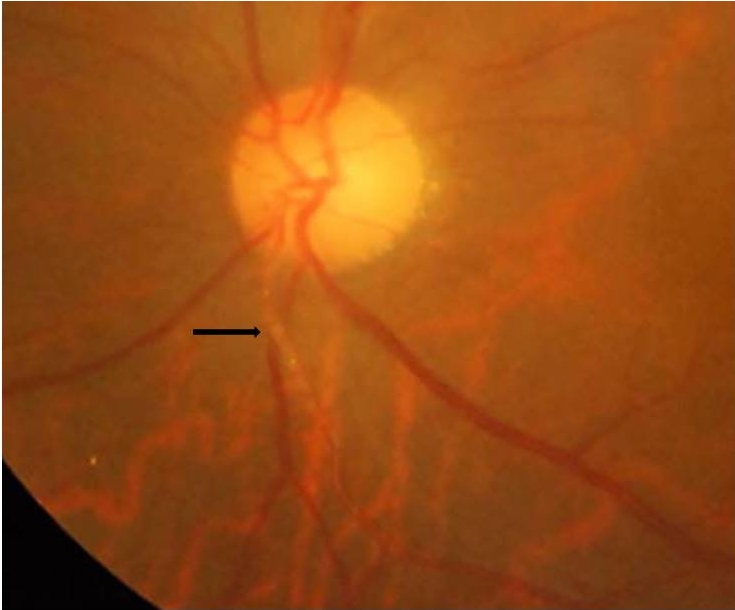
Vascular involvement can also occur either in the vicinity of the active lesion or in the distant retina and typically consists of a diffuse or segmental vasculitis(fig 6). It is produced by antigen-antibody complex deposition in the vessel wall. Vasculitis involves primarily the veins, but arterial involvement is also common.
Kyrieleis arteriolitis (fig 7) (the presence of exudates or periarterial plaques not associated with leakage or vascular obstruction) is also observed as an inflammatory response in ocular toxoplasmosis, and its pathogenesis is unknown[25].
The severity of anterior uveitis can range from mild anterior uveitis due to spill-over or intense anterior uveitis, masking the inflammation of the posterior segment. It can be either granulomatous or non-granulomatous inflammation. Intense anterior inflammation may occur secondary to retinochoroiditis, near the ora serrata, which may be missed at initial examinations [26,27].
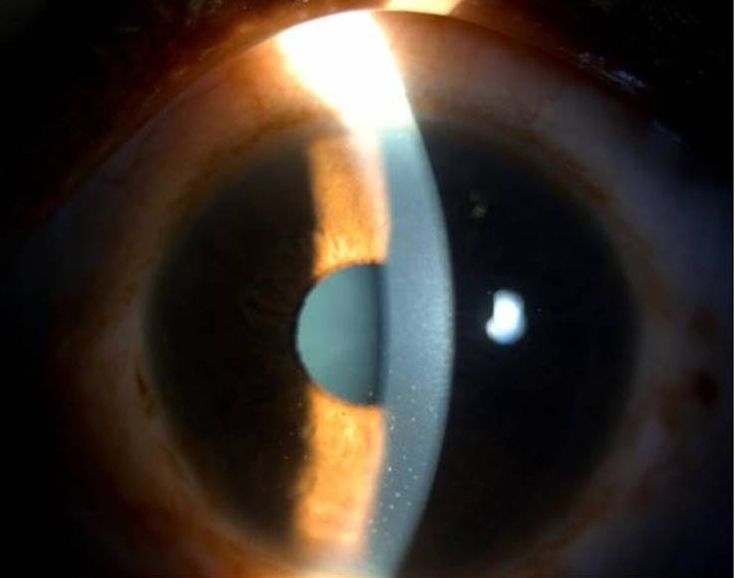
Ocular toxoplasmosis in immunocompromised patients
Immunocompromised patients including patients with human immunodeficiency virus (HIV), transplant recipients, and lymphoma patients[28,29] are at increased risk of developing severe fulminant infection. The disease may be caused by the reactivation of a chronic infection, or it may be an acquired infection.
Retinochoroiditis in these situations may have atypical features, like bilateral presentation, very large areas of severe confluent retinal necrosis, or multifocal lesions [30,31,32]. Lesions are usually perivascular in distribution, suggesting newly acquired infection or dissemination of parasites from other nonocular sites in the body[33]. The differential diagnosis of toxoplasmosis in immunocompromised may include cytomegalovirus (CMV) retinitis, syphilitic retinitis, and progressive outer retinal necrosis (PORN).
Atypical findings which can accompany retinochoroiditis[34]
- Papillitis
- Neuroretinitis
- Retrobulbar neuritis
- Scleritis
- Retinal detachment
- Punctate outer retinitis
- Branch retinal artery occlusion
- Frosted branch angiitis
- Fuchs’like anterior uveitis
- Multifocal diffuse necrotizing retinitis
Complications:
The most frequent complication is secondary glaucoma. Cataracts may occur secondary to severe vitreous inflammation or the use of local and systemic corticosteroids. The epiretinal membrane can cause macular puckering and cystoid macular edema. Other complications include choroidal neovascularization and branch retinal artery and vein occlusion, retinal detachment, and optic atrophy[35].
INVESTIGATIONS:
Ophthalmological investigations:
Fundus Autofluorescence:
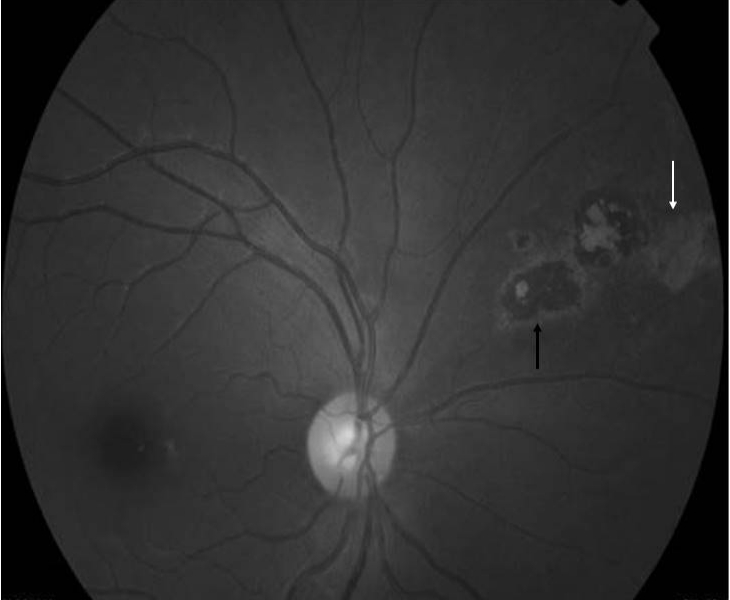
The active lesion on autofluorescence (AF) displays subtle hyper-AF or iso-AF that soon becomes hypo-AF at the rim and hyper-AF in the center as the healing starts. Hypo-AF rim progresses further centripetally until the complete absence of AF is seen.
Three distinct patterns of AF in toxoplasma retinochoroiditis have been described: (1) amorphous pattern: poorly defined area of hyper-AF corresponding to the retinitis lesion; (2) ring pattern: well-defined rim shaped area of hypo-AF around the central hyper-AF in the resolving stage; and (3) atrophic pattern: complete disappearance of hyper-AF.During reactivation, a well-defined hypo-AF corresponding to the scar and a poorly defined hyper-AF at the edge corresponding to an active retinitis lesion can be seen(36) (Fig 9).
Optical coherence tomography (OCT):
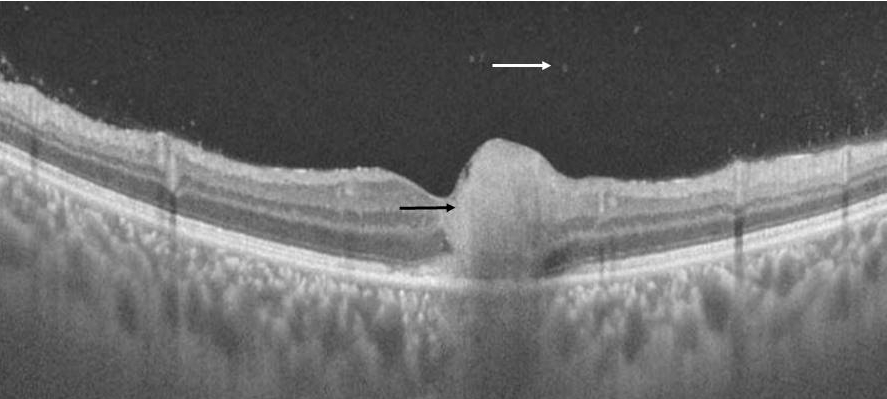
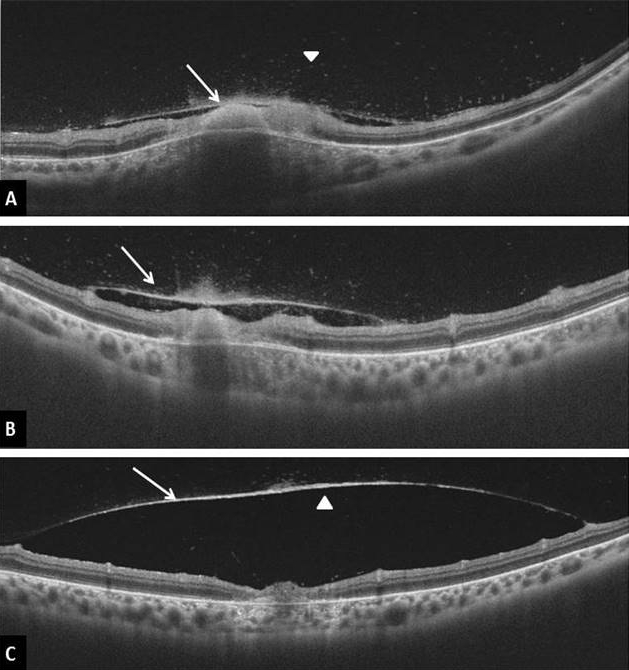
In the acute phase, disruption, thickening, and hyperreflectivity of the neurosensory retina with retinal pigment epithelial elevation is seen. During follow-up, neurosensory retinal layer thinning and disorganization, photoreceptor interruption, and retinal pigment epithelial atrophy are noted. Multiple hyperreflective dots in the vitreous cavity, compatible with cells, and posterior hyaloid thickening with partial detachment can also be noted in the acute phase[37,38,39]. An epiretinal membrane may be found overactive as well as scarred lesions ( fig10,11).
Fundus fluorescein angiography
FA findings include early hypofluorescence and late intense hyperfluorescence with fuzzy margins of the retinochoroiditis lesion in all active lesions [41]. In eyes with inactive lesions, the scar appeared hypofluorescent and the atrophic areas appeared hyperfluorescent on FA. FA also helps in identifying CNVM , involvement of optic nerve head, and leakage from the vessels(fig12).
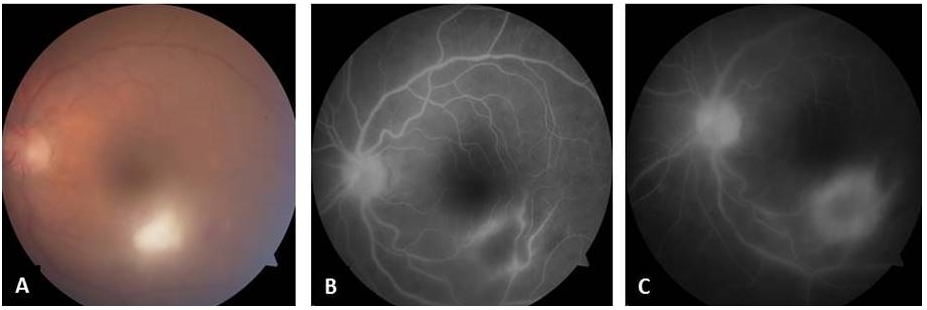
Indocyanine green (ICG)
ICGA is currently the best tool available to evaluate the choroid . Marked choroidal involvement is seen using ICGA, active lesion appears hypofluorescent and multiple hypofluorescent Satellite dark dots (SDD) are seen in 75% of patients with acute retinochoroiditis which disappear after treatment and can be observed in eyes with inactive lesions also [41,42]. Hyperfluorescent plaque is seen in presence of choroidal neovascularisation.
SEROLOGICAL INVESTIGATIONS
The ease of performance and the relatively high sensitivity and specificity of serologic methods make it gold standard for the diagnosis of toxoplasmosis. The detection of serum IgG and IgM by ELISA or immune fluorescent antibody kits is most commonly performed to confirm a clinically diagnosed ocular toxoplasmosis .
Toxoplasma-specific IgM detected in the serum is indicative of a recently acquired infection .Detection of Toxoplasma-specific IgG alone is of low diagnostic value as it indicates previous infection and is also found in individuals manifesting no signs of ocular toxoplasmosis[43,44].
Presence of IgG and IgM antibodies do not always indicate ocular infection.The common method used to estimate the local versus systemic Toxoplasma-specific IgG is the Goldmann-Witmer coefficient.
This index expresses the level of toxoplasma specific IgG in the aqueous humor in relation to the level of the total IgG in the serum.A value of 2 or above is generally taken as evidence of the intraocular synthesis of Toxoplasma- specific IgG in response to active ocular infection[45].
Polymerase Chain Reaction
Detection of parasitic DNA and toxoplasma specific antibodies in intraocular fluids(acqueous,vitreous) by polymerase chain reaction is considered highly sensitive and should be considered in all patients with atypical presentations and in patients not responding to anti toxoplasma therapy.This combined approach has diagnostic sensitivity and specificity of 60% and 90% respectively[46,47,48].
Immunoblotting
Immunoblotting indicates local antibody production in acqueous humor by detecting blot bands corresponding to IgG antibodies in major proportion, although IgM,IgA blot bands are also represented. The combined detection of IgA and IgG blot bands increases the specificity to 65%[49].
Treatment
- When to treat
- What treatment options are available
- Special situations ( Pregnancy, Immunosuppressed status)
When to treat:
Toxoplasma retinochoroiditis has a self limited course in immunocompetent individuals.
Indications for treatment[50]
a) The presence of a lesion within the vascular arcades of the posterior pole (zone 1)
b) The presence of a lesion in the proximity of the optic nerve or the macula
c) A severe inflammatory reaction within the eyes.
The zone 1 is an area extending 3000 μm (2 disk diameters) from the fovea (approximately that enclosed by the major temporal vascular arcades or 1500 μm from the margins of the optic disk.
d ) Multiple active lesions regardless of location
e ) Any active lesion in Immunocompromised host.
Medical treatment :
Anti parasitic drugs
The major aim of therapy is to stop the multiplication of tachyzoites during episodes of active retinochoroiditis. Ideal antiparasitic agent should be parasiticidal to both tachyzoites and bradyzoites and penetrate the cyst wall. It should be relatively safe and free of systemic side effects. There is no single agent with all these properties and no antibiotic is proven to be superior than any other.
A triple drug therapy is the classic treatment for Toxoplasma retinochoroiditis, consisting pyrimethamine, sulfadiazine and corticosteroids.
Pyrimethamine
It is an inhibitor of Dihydrofolate reductase enzyme, inhibits conversion of folic acid to folinic acid which is required for synthesis of both parasitic DNA and RNA. It has shown to reduce the size of retinochoroidalscar[ 51] and the healing time [ 48].
Dose: 100mg loading dose followed by 25mg/day for 30-60 days.
All patients must undergo periodic blood counts and should receive folinic acid 5-10mg/day (not folic acid) supplementation to prevent bone marrow suppression.
Sulfonamides
Sulfonamides are competitive inhibitors of Para amino benzoic acid (PABA) which is essential for folic acid synthesis by the parasite. It is synergistic with Pyrimethamine and has good intraocular penetration .As triple therapy Sulfadiazine with pyrimethamine and prednisolone is the drug of choice.[52] .Dose reduction should be considered in hepatic and renal failure as it causes crystalluria.
Dose: initial dose of 4 g daily for 2 days followed by 500 mg every 6 h for4-6weeks.
Trimethoprim Sulfamethoxazole
Trimethoprim and Sulfamethoxazole( 160 mg and800mg) twice daily for 30-40days are a reasonable alternative to Triple therapy as it is better tolerated[53].
Pyrimethamine, sulfadiazine is most effective but can cause bone marrow suppression and severe hepatotoxicity respectively. Hence,Trimethoprim ,Sulfamethoxazole and azithromycin are relatively safer options and reasonably effective, easily available and cost effective.
Azithromycin :Acts by reducing the ribosomal protein synthesis and is effective against bradyzoites (encysted form).
Dose: loading dose 1000mg for 3 days followed by 500 mg per day for 6 weeks.
Clindamycin: It is a lincosamide antibiotic, which concentrates in ocular tissues and penetrates tissue cyst walls. Clindamycin is often added to the classic triple therapy as part of the ‘quadruple therapy’[54]. Side effects include gastrointestinal disturbances and pseudomembranous colitis.
Dose- 300 mg every 6 hours for 30-40 days. In children 16-20 mg/ kg/day in divided doses 6 hourly.
Spiramycin: It is the drug of choice in pregnancy as it achieves higher placental concentration with no teratogenic effects[50].
Dose- 500 mg QID for 3 weeks and can be repeated in 3 weeks.
Atovaquone :It has a potent action against tachyzoites and has been shown to reduce tissue cysts in cerebral infection [55] .It is effective in acute infections and relatively safe option in AIDS patients.
Dose: 750 mg QID for 6-8 weeks.
Oral Corticosteroids
The addition of systemic corticosteroids to the therapeutic regimen may diminish the degree of collateral damage from the inflammatory response. Oral corticosteroids (0.5-1mg/kg/day ) is used in a tapering regimen for 5-6 weeks or even longer for large active lesions and prolonged intraocular inflammation. These are mainly indicated in vision threatening lesions in the posterior pole or close to the Optic nerve ,and in severe vitiritis. Corticosteroids are started 24-72 hours after starting anti microbials and tapered off before stopping them. Periocular and intravitreal steroids are contraindicated as a severe vision threatening inflammation can occur [56] .
Intravitreal treatment for toxoplasma retinochoroiditis
Intravitreal clindamycin (1mg-1.5 mg /0.1 ml) is also shown to be an effective and acceptable alternate to classic therapy [57,58].The advantage of Intravitreal clindamycin is its ability to achieve high local concentration and with fewer systemic side effects.
Indications of local therapy included intolerance to oral treatment, contraindication to systemic treatment because of pregnancy, lack of response despite systemic therapy, or combined oral and local treatment in sight-threatening cases to avoid or limit foveal or optic disc involvement
Treatment to reduce recurrence of toxoplasma retinochoroiditis
Use of trimethoprim sulfamethoxazole once in 2-3 days for 1 year following active episode for recurrent cases of toxoplasma retinochoroiditis, in the absence of contraindications reduces the recurrence rate [59].
Special situations
Pregnancy
Factors to be concerned during treatment are teratogenecity of antimicrobials during pregnancy, transplacental transmission of the parasite causing congenital toxoplasmosis (fetal anomalies and neurological defects) and to determine whether the infection is a primary infection or recurrent /reactivation of previous infection.
Table1 : Approach in pregnancy based on serology [60]
|
IgG/IgM Essentially Performed in 1st trimester |
IgG (-) IgM (+) |
No evidence of prior infection.Avoid exposure during pregnancy |
Testing at follow up visits to see seroconversion.If detected treat as IgG+ve/IgM+ve |
|
|
IgG (+) IgM (-) |
<18 weeks gestation –indicates past infection. Risk for CT is low. |
No further action required |
||
|
>18 weeks gestation- difficult to establish if infection occurred in past or during pregnancy |
Attempt to obtain earlier sera results. |
|||
|
IgG (–) IgM (+) |
Repeat IgG,IgM in 1-3 weeks |
IgG(-),IgM(+) - has no clinical relevance |
Management is as IgG –ve, IgM -ve |
|
|
IgG (+)IgM(+) – fetus is at risk for CT |
Treatment to be initiated.amniotic PCR at 18 weeks or as soon thereafter as feasible.fetal ultrasound to be considered. |
|||
|
IgG (+) IgM (+) |
Serum to be sent to reference laboratory for confirmatory testing |
|||
Cases of peripheral lesion with minimal inflammation can be observed (self-limiting in immunocompetent), if toxoplasma infection is not acquired during pregnancy.
Zone 1 lesions should be treated with Spiramycin as it has minimal/ negligible transplacental transmission.
Dose: 500 mg every 6 hours or 3 lakh IU/ every 8 hours( 1 gm tid) for 10 days. If not improving with Spiramycin and the patient is beyond the first trimester (16 weeks ) sulfadiazine and pyrimethamine with Folinic acid supplement can be given for ocular toxoplasmosis.
Immunocompromised situations
Challenges in Immunocompromised cases are Atypical presentation of the disease with less inflammation, rapid progression, bilateral presentation. Serological diagnosis may not be accurate and Molecular diagnostic methods ( PCR analysis) are helpful[61].
Any active retinal lesion in an immunocompromised patient needs to be treated because of high risk of disseminated disease ( encephalopathy) and its complications. The triple therapy regimen or trimethoprim sulfamethoxazole combination can be used, and is found effective .Treatment is given until 4–6 weeks after resolution of all signs and symptoms.The disease frequently recurs when medical treatment is discontinued and requires prolonged maintenance therapy with Atovaquone 750mg TID or Clarithromycin 500mg BD.
Corticosteroids are to be avoided due to high risk of CMV retinitis. Intravitreal clindamycin achieves high concentration in vitreous and is free of systemic side effects, can be used for vision threatening rapidly progressive retinitis. CNS toxoplasmosis is a common association with ocular toxoplasmosis and has to be investigated[62].
Conclusion
Ocular toxoplasmosis is the most common cause of infectious posterior uveitis, and its diagnosis in the majority of the cases is made clinically by the presence of intense vitritis, satellite retinitis lesions next to a healed retinochoroidal scar. Laboratory confirmation is by antibody detection through ELISA,or by PCR to identify parasitic DNA. Treatment consists of classical triple drug therapy of pyrimethamine, sulphadiazine and corticosteroid. Alternative effective treatment regimens include trimethoprim, sulphamethaxazole, azithromycin with pyrimethamine or intravitreal clindamycin along with oral therapy.
References
- Henderly, D. E., Genstler, A. J., Smith, R. E., & Rao, N. A. (1987). Changing patterns of uveitis. American journal of ophthalmology, 103(2), 131–136.
- Smith RE, Nozik RA (1989) Uveitis: A Clinical Approach to Diagnosis and Management, 2nd ed. Baltimore, Williams & Wilkins.
- Jabs D. A. (1990). Ocular toxoplasmosis. International ophthalmology clinics, 30(4), 264–270.
- Fernandes, L. C. (1995). Aspectos clínicos e epidemiológicos das uveítes, em serviços de referência em Belo Horizonte, de 1970 a 1993.
- Perkins ES. Ocular toxoplasmosis. Br J Ophthalmol 1972; 57: 1-17.
- Glasner, P. D., Silveira, C., Kruszon-Moran, D., Martins, M. C., Burnier Jr, M., Silveira, S. & Belfort Jr, R. (1992). An unusually high prevalence of ocular toxoplasmosis in southern Brazil. American journal of ophthalmology, 114(2), 136-144.
- Holland, G. N. (2000). Ocular toxoplasmosis: new directions for clinical investigation. Ocular Immunology and Inflammation, 8(1), 1-7.
- Atmaca, L. S., Simsek, T., & Batioglu, F. (2004). Clinical features and prognosis in ocular toxoplasmosis. Japanese journal of ophthalmology, 48(4), 386-391.
- Chowdhury M. N. (1986). Toxoplasmosis: a review. Journal of medicine, 17(5-6), 373–396.
- Robert-Gangneux, F., & Dardé, M. L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical microbiology reviews, 25(2), 264-296.
- Shimada, K., O'Connor, G. R., & Yoneda, C. (1974). Cyst formation by Toxoplasma gondii (RH strain) in vitro: The role of immunologic mechanisms. Archives of ophthalmology, 92(6), 496-500.
- Lune, A. A., Pujari, S. N., & Lune, S. A. (2014). Ocular toxoplasmosis: A case report with review of literature. Medical Journal of Dr. DY Patil University, 7(6), 818.
13.C Stephen Foster ,Albert T Vitale(2013).Diagnosis and treatment of uveitis; Toxoplasmosis,2nd edition;New Delhi;Jaypee;2013.p 543-568.
14. Brézin, A. P., Kasner, L., Thulliez, P., Li, Q., Daffos, F., Nussenblatt, R. B., & Chan, C. C. (1994). Ocular toxoplasmosis in the fetus. Immunohistochemistry analysis and DNA amplification. Retina (Philadelphia, Pa.), 14(1), 19-26.
15. Silveira, C., Belfort Jr, R., Burnier Jr, M., & Nussenblatt, R. (1988). Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. American journal of ophthalmology, 106(3), 362-364.
16. De Moura, L., Bahia-Oliveira, L. M. G., Wada, M. Y., Jones, J. L., Tuboi, S. H., Carmo, E. H., ... & Garrett, D. O. (2006). Waterborne toxoplasmosis, Brazil, from field to gene. Emerging infectious diseases, 12(2), 326.
17. Palanisamy, M., Madhavan, B., Balasundaram, M. B., Andavar, R., & Venkatapathy, N. (2006). Outbreak of ocular toxoplasmosis in Coimbatore, India. Indian journal of ophthalmology, 54(2), 129-131.
18. Gilbert, R. E., & Stanford, M. R. (2000). Is ocular toxoplasmosis caused by prenatal or postnatal infection?. British journal of ophthalmology, 84(2), 224-226.
19. Nussenblatt, R. B. (1989). Uveitis. Fundamentals and Clinical Practice, 336.
20 . McLeod R, Remington JS (1987) Toxoplasmosis. Harrison's Principles of Internal Medicine, 11th ed. New York, McGraw-Hill, pp 791-797.
21. Suhardjo, U. P., & Agni, A. N. (2003). Clinical manifestations of ocular toxoplasmosis in Yogyakarta, Indonesia: a clinical review of 173 cases. Southeast Asian J Trop Med Public Health, 34(2), 291-7.
22 Brady-McCreery, K. M., Hussein, M. A., & Paysse, E. A. (2003). Congenital toxoplasmosis with unusual retinal findings. Archives of Ophthalmology, 121(8), 1200-1201.
23. stephen, Foster C; Vitale, Albert T. (2002). Diagnosis and Treatment of Uveitis. W.B. Saunders Company.ch 33 ,p358-410
24 Park, Y. H., & Nam, H. W. (2013). Clinical features and treatment of ocular toxoplasmosis. The Korean journal of parasitology, 51(4), 393.
25. Rodenhäuser, J. H. (1969). Clinical findings and pathogenesis of Kyrieleis' discontinuous reversible arteriopathy in uveitis. Klinische Monatsblatter fur Augenheilkunde, 155(2), 234-243
26 Islam, S. M., & Tabbara, K. F. (2002). Causes of uveitis at The Eye Center in Saudi Arabia: a retrospective review. Ophthalmic epidemiology, 9(4), 239-249.
27. Holland, G. N., Engstrom, R. E., Jr, Glasgow, B. J., Berger, B. B., Daniels, S. A., Sidikaro, Y., Harmon, J. A., Fischer, D. H., Boyer, D. S., & Rao, N. A. (1988). Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. American journal of ophthalmology, 106(6), 653–667.
28. Cohen, S. N. (1970). Toxoplasmosis in patients receiving immunosuppressive therapy. Jama, 211(4), 657-660.
29. Araujo, F. G., & Remington, J. S. (1987). Toxoplasmosis in immunocompromised patients. European journal of clinical microbiology, 6(1), 1-2.
30 Holland, G. N. (1989). Ocular toxoplasmosis in the immunocompromised host. International ophthalmology, 13(6), 399-402.
31. Schuman, J. S., & Friedman, A. H. (1983). Retinal manifestations of the acquired immune deficiency syndrome (AIDS): cytomegalovirus, candida albicans, cryptococcus, toxoplasmosis and Pneumocystis carinii. Transactions of the ophthalmological societies of the United Kingdom, 103 ( Pt 2), 177–190.
32. Heinemann, M. H., Gold, J. M., & Maisel, J. A. M. E. S. (1986). Bilateral toxoplasma retinochoroiditis in a patient with acquired immune deficiency syndrome. Retina (Philadelphia, Pa.), 6(4), 224-227.
33. Momthy RS, RaoNA(1997) Posterior uveitis. Wright KW: Textbook of Ophthalmology ;New York: Williams & Wilkins.
34. Smith, J. R., & Cunningham, E. T. (2002). Atypical presentations of ocular toxoplasmosis. Current opinion in ophthalmology, 13(6), 387-392.
35. Park, Y. H., & Nam, H. W. (2013). Clinical features and treatment of ocular toxoplasmosis. The Korean journal of parasitology, 51(4), 393.
36. Brandão-de-Resende, C., Balasundaram, M. B., Narain, S., Mahendradas, P., & Vasconcelos-Santos, D. V. (2020). Multimodal imaging in ocular toxoplasmosis. Ocular Immunology and Inflammation, 28(8), 1196-1204.
37 Alwassia, A. A., Cho, H., Adhi, M., Duker, J. S., & Baumal, C. R. (2013). Sequential optical coherence tomography images of retinal necrosis in acute ocular toxoplasmosis. Retinal Cases & Brief Reports, 7(1), 98-101.
38 Goldenberg, D., Goldstein, M., Loewenstein, A., & Habot-Wilner, Z. (2013). Vitreal, retinal, and choroidal findings in active and scarred toxoplasmosis lesions: a prospective study by spectral-domain optical coherence tomography. Graefe's Archive for Clinical and Experimental Ophthalmology, 251(8), 2037-2045.
39 Oréfice, J. L., Costa, R. A., Scott, I. U., Calucci, D., Oréfice, F., & Grupo Mineiro de Pesquisa em Doenças Oculares Inflamatórias (MINAS). (2013). Spectral optical coherence tomography findings in patients with ocular toxoplasmosis and active satellite lesions (MINAS Report 1). Acta ophthalmologica, 91(1), e41-e47.
40. Ozgonul, C., & Besirli, C. G. (2017). Recent developments in the diagnosis and treatment of ocular toxoplasmosis. Ophthalmic research, 57(1), 1-12.
41. Atmaca, L. S., Simsek, T., Atmaca Sonmez, P., & Sonmez, K. (2006). Fluorescein and indocyanine green angiography in ocular toxoplasmosis. Graefe's Archive for Clinical and Experimental Ophthalmology, 244(12), 1688-1691.
42. Auer, C., Bernasconi, O., & Herbort, C. P. (1999). Indocyanine green angiography features in toxoplasmic retinochoroiditis. Retina (Philadelphia, Pa.), 19(1), 22-29.
43. Holliman, R. E., Stevens, P. J., Duffy, K. T., & Johnson, J. D. (1991). Serological investigation of ocular toxoplasmosis. The British Journal of Ophthalmology, 75(6), 353.
44. Chapman, D. J., Ashburn, D., Ogston, S. A., & Ho-Yen, D. O. (1999). The relationship between ocular toxoplasmosis and levels of specific toxoplasma antibodies. Epidemiology & Infection, 122(2), 299-303.
45. Turunen, H. J., Leinikki, P. O., & Saari, K. M. (1983). Demonstration of intraocular synthesis of immunoglobulin G toxoplasma antibodies for specific diagnosis of toxoplasmic chorioretinitis by enzyme immunoassay. Journal of Clinical Microbiology, 17(6), 988-992.
46 Garweg, J. G., de Groot-Mijnes, J. D., & Montoya, J. G. (2011). Diagnostic approach to ocular toxoplasmosis. Ocular immunology and inflammation, 19(4), 255-261.
47 Wakefield, D., Cunningham Jr, E. T., Pavesio, C., Garweg, J. G., & Zierhut, M. (2011). Controversies in ocular toxoplasmosis. Ocular immunology and inflammation, 19(1), 2-9.
48 Villard, O., Filisetti, D., Roch-Deries, F., Garweg, J., Flament, J., & Candolfi, E. (2003). Comparison of enzyme-linked immunosorbent assay, immunoblotting, and PCR for diagnosis of toxoplasmic chorioretinitis. Journal of Clinical Microbiology, 41(8), 3537-3541.
49. Garweg, J. G., Garweg, S. D. L., Flueckiger, F., Jacquier, P., & Boehnke, M. (2004). Aqueous humor and serum immunoblotting for immunoglobulin types G, A, M, and E in cases of human ocular toxoplasmosis. Journal of Clinical Microbiology, 42(10), 4593-4598.
50. Guex-Crosier Y. (2009). Update on the treatment of ocular toxoplasmosis. International journal of medical sciences, 6(3), 140–142.
51 Rothova, A., Meenken, C., Buitenhuis, H. J., Brinkman, C. J., Baarsma, G. S., Boen-Tan, T. N., ... & Kijlstra, A. (1993). Therapy for ocular toxoplasmosis. American journal of ophthalmology, 115(4), 517-523.
52 Nolan, J., & Rosen, E. S. (1968). Treatment of active toxoplasmic retino-choroiditis. The British journal of ophthalmology, 52(5), 396.
53. Opremcak, E. M., Scales, D. K., & Sharpe, M. R. (1992). Trimethoprim-sulfamethoxazole therapy for ocular toxoplasmosis. Ophthalmology, 99(6), 920-925.
54. Baharivand, N., Mahdavifard, A., & Fouladi, R. F. (2013). Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. International ophthalmology, 33(1), 39-46.
55 . Gormley, P. D., Pavesio, C. E., Minnasian, D., & Lightman, S. (1998). Effects of drug therapy on Toxoplasma cysts in an animal model of acute and chronic disease. Investigative ophthalmology & visual science, 39(7), 1171-1175.
56 Bosch-Driessen, L. E., Berendschot, T. T., Ongkosuwito, J. V., & Rothova, A. (2002). Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology, 109(5), 869-878.
57. Sobrin, L., Kump, L. I., & Foster, C. S. (2007). Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina, 27(7), 952-957.
58 Lasave, A. F., Díaz-Llopis, M., Muccioli, C., Belfort Jr, R., & Arevalo, J. F. (2010). Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology, 117(9), 1831-1838.
59. Felix, J. P., Lira, R. P., Zacchia, R. S., Toribio, J. M., Nascimento, M. A., & Arieta, C. E. (2014). Trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: randomized controlled clinical trial. American journal of ophthalmology, 157(4), 762–766.
60. Montoya, J. G. (2004). Liesenfeld, O1: CAS: 528: DC% 2BD2cXkvVemuro% 3D. vol. 363. Toxoplasmosis Lancet, 1965-1976.
61. Hazan, A., Patel, R. M., Levinson, D., Mian, U., & Gritz, D. C. (2013). A typical bilateral Toxoplasma retinochoroiditis in a bone marrow transplant patient with negative serum titers. Journal of ophthalmic inflammation and infection, 3(1), 1-4.
62. Holland G. N. (1989). Ocular toxoplasmosis in the immunocompromised host. International ophthalmology, 13(6), 399–402.