Endophthalmitis is a potentially devastating ocular disease that may lead to permanent loss of vision. It is caused by bacteria in the majority of the cases. However, fungal endophthalmitis though rare has a poor prognosis and usually diagnosed late. The incidence of fungal endophthalmitis has increased in recent years, particularly in developing countries. 1-3Unlike bacterial infections, we do not have much choice of medications in fungal infections. The fungus is slow-growing and we have limited choice of antifungalswhich act slowly. These are usually diagnosed late as patients are less symptomatic but have more signs. Moreover, fungal culture takes longer time and identification requires more expertise. Polymerase chain reaction (PCR) techniques can even detect dead remnants. Patients are usually treated as bacterial infections and would have received steroids before they are diagnosed to have fungal endophthalmitis. The prognosis of fungal endophthalmitis depends on the magnitude of intraocular involvement, the virulence of the organism, and the timing of treatment. Early diagnosis of fungal endophthalmitis is important in order to prevent irreversible damage to the retina and prevent visual loss.
Classification:
The fungus can be classified into yeasts, molds, and dimorphic. Yeasts include candida, cryptococcus, trichosporon, and rhodotorula. Molds can further be classified based on hyphae as aseptate (rhizopus and mucor) and sepate (aspergillus, fusarium, dermatophytes such as trichophyton and microspore). Dimorphic fungi include Histoplasma, Blastomyces, coccidiosis, and sporothrix.
Antifungal drugs can be classified based upon structure and their mechanism of action.
|
1.Antibiotics |
||
|
a.Polyene |
Amphotericin Nystatin Natamycin |
Binds irreversible to ergosterol in the cell membrane and creates transmembrane channel and electrolyte leakage leading to fungal cell death |
|
b.Heterocyclic bezofuran |
Griseofulvin |
Disruption of mitotic spindle and inhibition of fungal mitosis |
|
c.Echinocandins |
Capsofungin Micafungin Anidulafungin |
Inhibition of fungal cell wall synthesis |
|
2.Antimetabolite |
Flucytosine |
Inhibition of nucleic acid synthesis |
|
3.Azoles |
||
|
a.Imidazoles |
Ketoconazole |
Inhibition of ergosterol synthesis disrupts cell membrane function and increases permeability |
|
b.Triazoles |
Fluconazole Itraconazole Voriconazole |
Inhibition of ergosterol synthesis disrupts cell membrane function and increases the permeability |
Fungal endophthalmitis can be classified into exogenous and endogenous types.
Exogenous Endophthalmitis
It occurs due to the direct inoculation of fungus following trauma or intraocular surgery. Source of pathogens is the ocular surface (e.g., in postoperative, keratitis-related, and bleb-related endophthalmitis) or the environment (e.g., in posttraumatic endophthalmitis). It usually occurs in immunocompetent patients. A study by Williamson et al have reported that the overall incidence of fungi was 2.9% in patients with healthy conjunctival flora. 4Fungal cultures comprised of Penicillium Sp., Aspergillus Sp., Rhodotorula Sp., Scopulariopsis Sp., and Candida Sp. in the majority of the cases. 4The incidence of fungal isolates increases in older age, prolonged steroid use, and patients with Sjogren's syndrome. 4,5The frequency of endophthalmitis after cataract surgery and trauma ranges from 0.07% to 0.13% and 2.4 to 17%, respectively, as previously reported in the literature. 6In a retrospective study of 170 culture-positive postoperative endophthalmitis cases in India, 21.8% of cases were due to fungi.7The postoperative fungal endophthalmitis is usually seen due to use of contaminated intraocular irrigation solution, intraocular lenses, ventilation system, hospital construction activities or surgeries conducted in rural settings or eye camps. 8Fusarium and Aspergillus species are the most common causative organisms. (9,10) In the studies from India, Aspergillus species are found to be most common. 2,3,7,8In a study by Chakrabarti et al, Aspergillus species were the most common (54.4%), followed by yeasts (24.6%) and melanized fungi (10.5%). 8Among Aspergilli, Aspergillus flavus was the most common (24.6%) whereas Candida tropicalis (8.8%) was most common in the yeast. Other rare agents included Fonsecaea pedrosoi, Fusarium solani, Paecilomyces lilacinus, Pseudallescheria boydii, Colletotrichum dematium, Cryptococcus neoformans, and Trichosporon cutaneum.
Endogenous Endophthalmitis
It arises from the hematogenous seeding of pathogens during fungemia. Most patients with endogenous fungal endophthalmitis have predisposing systemic risk factors, such as systemic debilitating disease, uncontrolled diabetes mellitus, intravenous drug use, recent hospitalization, parenteral nutrition, central venous catheter, malignancy, systemic surgeries especially abdominal surgery, an immunocompromised status due to HIV infection, neutropenia or prolonged steroid and immunosuppressant therapy for organ transplantation; liver disease, pulmonary disease or renal failure. 11-14It has also been described in healthy patients after receiving presumably contaminated dextrose infusions. 15, 16The most common causative organisms are Candida species. 8, 11, 17In a study on endogenous endophthalmitis conducted in southern India, Pseudomonas was the most common organism (13.8%), followed by Candida (8.6%) and E. coli (6.9%). 18Children and adolescents can also get affected by fungal infections like Candida and Aspergillus. Neonatal Candida endogenous endophthalmitis has been reported secondary to neonatal sepsis. The incidenceof endogenous endophthalmitis in the pediatric age group is higher in India that could be due to general malnutrition which reduces the immunity, thus making children more prone to latent infections to become manifest.
Clinical features:
The patient usually presents with diminution in vision, pain, redness, and lid edema. Systemic symptoms such as fever or malaise are often present in patients with endogenous endophthalmitis.19On anterior segment examination, corneal edema, anterior chamber cellular reaction, posterior synechiae, fibrin or hypopyon is seen in most of the cases. Yellowish white nodular exudates are usually seen on the iris and lens surface. Hypopyon is nodular and organized. Fundus examination shows media haze due to vitreous exudates that often obscures the view of retinal vessels. It is important to differentiate between bacterial and fungal endophthalmitis. Bacterial endophthalmitis usually presents acutely, often within days of an inciting event such as cataract surgery or trauma. Fungal endophthalmitis typically has a subacute presentation with symptoms worsening over days to weeks. The symptoms are less severe in fungal endophthalmitis. The visual deterioration and pain are significantly lesser in fungal endophthalmitis. The patient presents with more signs. The intraocular inflammation in fungal endophthalmitis tends to be more localized and occurs in clumps while it is typically diffuse in bacterial endophthalmitis. The clinical picture may vary depending upon the etiologic agent. Fundus examination shows fluffy white focal areas of chorioretinal lesions with overlying vitritis or cotton balls may be suspended in the vitreous with “string of pearls” appearance in cases with Candida endophthalmitis. 10However in cases with Aspergillus endophthalmitis, a confluent yellowish infiltrate is often seen in the macular area, beginning in the choroid and subretinal space. It has an acute presentation and there can be associated retinal hemorrhages, retinal vascular occlusions, and full-thickness retinal necrosis. 20Endophthalmitis should be differentiated from other similar looking conditions such as acute retinal necrosis, progressive outer retinal necrosis, degenerated cysticercus cyst 21, cytomegalovirus retinitis toxoplasmosis, Behcet’s disease, syphilis, intraocular tumors, and other masquerade syndromes. In a study by Maitray et al, they reported differences in endogenous endophthalmitis among children and adolescents from that in adults to include the relative lack of systemic features and lack of underlying systemic disorders as well as potential misdiagnosis as masquerade syndrome.22Patient may present as panophthalmitis with marked lid edema, proptosis, and limitation of extraocular movements. Details of the anterior and posterior segments are not visible because of the prominent hypopyon. This rapidly developing infection invades the orbit leading to blindness, phthisis, or enucleation. Progression of a panophthalmitis may be life-threatening. Rishi et al has reported a study on endophthalmitis in eyes presenting with orbital signs (defined as ocular motility restriction and/or presence of "inverted perpendicular" sign on ultrasonography) and found out that orbital signs are independent risk factors for poor structural and visual outcomes in eyes with endophthalmitis. 23
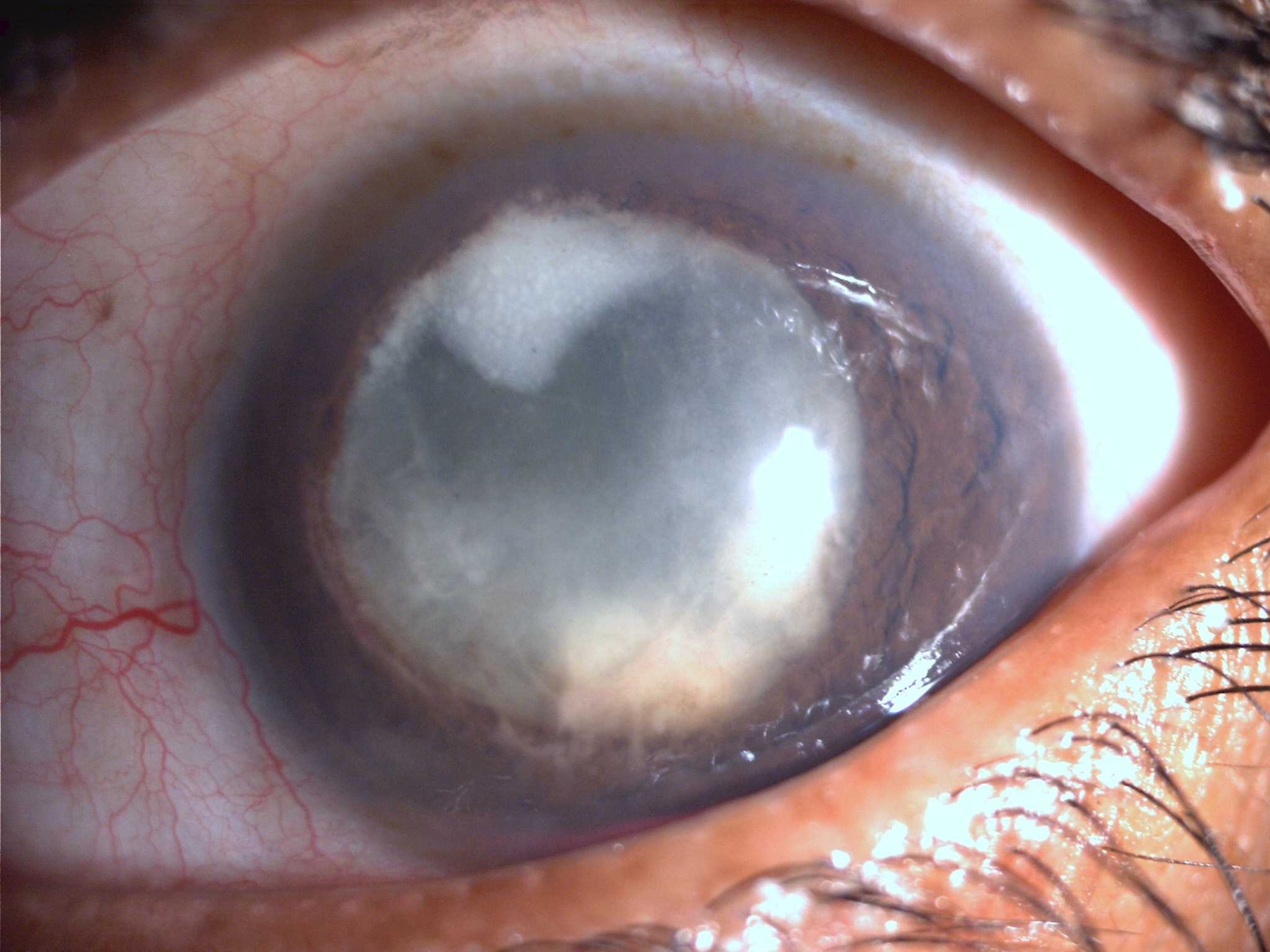 Figure 1: Colour Slit-lamp photograph of Left eye with endogenous Fungal Endophthalmitis. The patient has extensive exudates behind and in front of the crystalline lens with relatively less congestion.
Figure 1: Colour Slit-lamp photograph of Left eye with endogenous Fungal Endophthalmitis. The patient has extensive exudates behind and in front of the crystalline lens with relatively less congestion.
Management:
Ultrasonography:
It should be done to look for the extent of inflammation, vitreous membranes and opacities, presence and location of posterior vitreous detachment, presence of retinal detachment, choroidal detachment, or T sign, any choroidal abscess, any retained lens matter or intraocular foreign body and retinochoroidal thickening. It helps in monitoring response to treatment and rules out other masquerades like cysticercus cyst or intraocular tumors.
Aqueous tap/ Vitreous tap or biopsy:
After cleaning the eye and the periocular skin with 5% povidone-iodine and applying a wire speculum, a 30-gauge needle mounted on a 1 mL disposable syringe can be used for aspirating approximately 0.1-0.2 mL of aqueous humor. A vitreous sample may be obtained either by a needle tap or by vitreous biopsy. In a vitreous tap, a 27-gauge needle with 2 mL or 5 mL syringe is passed through pars plana and 0.2-0.3 mL of undiluted vitreous is removed avoiding forceful suction. Some vitreous aspirates are “dry taps” because of the difficulty in aspirating a gel. There is a possibility of vitreous traction and subsequent retinal detachment in non-vitrectomized eyes. Vitreous samples can be collected during planned therapeutic vitrectomy. The aspiration line can be connected to a 10 mL disposable syringe and gentle suction should be applied while the surgeon actuates the vitrectomy cutter. During this process, the infusion line should be kept blocked to avoid dilution of the vitreous sample. The vitreous samples collected during vitrectomy are known to yield higher culture-positive results than a needle biopsy of the vitreous. (24) Aqueous aspirates usually have higher yields in endophthalmitis cases in which inflammation is greatest in the aqueous, such as cases secondary to keratitis. 25The aqueousor vitreous sample is subjected to microbiological analysis. Samples can be sent for Gram stain, 10% KOH mount, Giemsa stain, and Ziehl-Neelsen stain to identify bacterial or fungal etiology. All samples should be inoculated on blood agar, chocolate agar, MacConkey agar, Sabouraud dextrose agar, thioglycolate medium, brain-heart infusion agar, and Lowenstein-Jensen agar. All samples must be inoculated in Sabaraud's dextrose agar media for at least six weeks at 250C and 370C respectively.3A positive culture is defined as the growth of the same organism on two or more solid phase media or confluent growth on one solid medium. Antimicrobial sensitivity patterns can be recorded using the Kirby-Bauer disc diffusion method. Cultures are positive in approximately 90% of vitrectomy samples, 50 to 70% of vitreous aspirates, and 40% of aqueous aspirates. (25) Culture rates are lower in fungal endophthalmitis.
Molecular diagnostic techniques:
PCR testing of aqueous and vitreous samples can rapidly identify pathogens in endophthalmitis cases, including culture-negative cases. In fungal infections, even when cultures are positive, results usually take longer than a week because these organisms are difficult to identify and/or are slow-growing. Early diagnosis and rapid intervention are required for effective treatment of these ocular infections. Multicopy gene targets have been evaluated for increasing the sensitivity for the detection of fungal pathogens and universal fungal PCR primers have been developed for broadening the range of detectable fungi. 26,27Ferrer et al have reported on rapid detection and identification of fungal pathogens by PCR and amplification of ITS2 and 5.8S ribosomal DNA and molecular typing. 28Gandhi et al have evaluated the clinical utility of high-throughput sequencing approach-based analysis in identifying predominantly fungal genome in vitreous fluids after DNA extraction and amplification of ITS 2 region in patients with clinically infectious culture-negative endophthalmitis. 29
Blood/Urine culture:
In cases of endogenous endophthalmitis, blood and urine cultures should be done. Blood cultures are often positive in these patients but may be negative in cases due to transient fungemia, such as those related to intravenous drug use, an indwelling central venous catheter, or an outpatient gastrointestinal procedure. Blood cultures are reported to be more likely positive than vitreous in a large series of endogenous endophthalmitis. 19,30,31
Treatment:
The mainstay of treatment for postoperative fungal endophthalmitis is the intravitreal injection of antifungal agents [amphotericin (5 ug/0.1mL) or voriconazole (50 ug /0.1 mL)] and surgical debulking. Intravitreal injections can be repeated after 48 hours if a patient does not show improvement after the first injection or if there is any deterioration. Amphotericin B has been the drug of choice for a long time. It is a broad spectrum efficacious fungicidal drug. A combination of vitrectomy and antifungal agents appears to be the best therapy for fungal endophthalmitis.14,19,31-35Vitrectomy debulks the infective tissue and may help in improving the diffusion of antifungal agents across the vitreous cavity, retina, and choroid.
The role of steroids in the treatment of fungal endophthalmitis is controversial. The size of fungal hyphae may preclude ingestion by the neutrophils, leading to the release of lysosomal enzymes and oxygen metabolites into the surrounding tissues. Steroids are often used to control the surrounding tissue destruction due to direct damage by fungal toxins and by host defense mechanisms. The use of steroids, as anti-inflammatory agents, in a case of fungal endophthalmitis has been reported in various studies.36–40Adverse effects of steroids are reported mainly due to high intravitreal dosage and if they are not combined with an efficacious antifungal agent. 41-43Systemic antifungals agents such as itraconazole 100 mg BD, voriconazole 200 mg BD or fluconazole 200 mg BD should be given in these cases. Liver function tests should be repeated at regular intervals to monitor hepatic toxicity. Topical antifungal agents should be added in case of corneal involvement. Natamycin has good efficacy against filamentous fungi but does not penetrate well into the cornea. Topical amphotericin B is commonly used in the management of fungal keratitis. Fungal endophthalmitis poorly responds to medication and early vitrectomy should be considered.
In all cases of endogenous endophthalmitis, intravenous antifungal agents are initially administered along with systemic antifungal agents. Post-traumatic endophthalmitis may be associated with an injury with vegetative matter and may require vitrectomy to debulk the fungal load and increase the penetration of antifungal drugs. Postoperative fungal endophthalmitis may require early vitrectomy along with the removal of the intraocular lens and capsular bag. Patients with significant vitritis and macula threatening lesions usually require vitrectomy along with intravitreal antifungal injections (amphotericin or voriconazole) in addition to systemic therapy. For systemic agents, fluconazole is recommended for fluconazole-susceptible Candida, voriconazole for fluconazole-resistant but voriconazole-susceptible isolates, and liposomal amphotericin, with or without 5-flucytosine, for azole-resistant strains. The advantage of voriconazole over fluconazole is that it has activity against Aspergillus species and fluconazole-resistant Candida species, such as Candida glabrata and Candida krusei. Low intraocular levels are attained with systemic administration of amphotericin B and it also carries the risk of infusion toxicity and nephrotoxicity. 44Intravitreal amphotericin B can cause retinal toxicity and necrosis if given in high doses. Ganglion cell damage and retinal detachment are reported secondary to increased membrane permeability induced by amphotericin B. 45Voriconazole may be safer than amphotericin B. It has excellent bioavailability, less toxic and immediately achieves high levels of the drug in the vitreous, whereas serum levels from systemic administration are gradually reaching a steady state. Clinical efficacy of systemic voriconazole has been reported in Aspergillus, Fusarium, and Candida endophthalmitis. The dose of intravenous fluconazole (12 mg/kg loading dose, then 6–12 mg/kg daily) or voriconazole (6 mg/kg every 12 hours (24 hours), then 4 mg/kg every 12 hours) can be used. Krishnan et al has reported the role of intravitreal voriconazole in the management of neonatal candidal endogenous endophthalmitis. 46There is poor ocular penetration with newer antifungal agents such as posaconazole and echinocandins (micafungin, caspofungin, and anidulafungin). 47-50Guest et al has reported a significant improvement in Aspergillus endophthalmitis in mice treated with isavuconazole, with a considerable reduction in both fungal burden and intraocular inflammation. 51
The nature of fungus is an important prognostic factor for visual outcomes. Aspergillus infection has been associated with a poor outcome with a higher chance of evisceration compared to Candida infection. Corneal involvement in addition to endophthalmitis and the presence of Aspergillus terreus were found to be poor prognostic markers.52Prognosis of fungal endophthalmitis with concurrent retinal detachment is usually poor with severe visual impairment. Silicone oil has antimicrobial properties and found to be helpful for better anatomical and functional results in endophthalmitis.53,54However, silicone oil might play a weaker role in antifungal therapy. 55It has been found that nontoxic concentrations of intravitreal injections create toxicity in a silicone oil-filled eye. 56Post cataractfungal endophthalmitisnearly always requiresremovalof the intraocular lens (IOL)in addition to antifungals and vitrectomy. Early debulking is important. The fungus may survive over the IOL surface and in the capsular bag and can cause recurrences. Vinekar et al. has described the role of combined IOL explantation with capsulectomy and re-vitrectomy in the management of recurrent postoperative fungal endophthalmitis after failed vitrectomy and antifungal therapy. 57 Due to poor penetration of systemic and topical medications and recurrent need for vitrectomy to debulk the load of fungus, there is a high possibility of eye landing up in phthisis despite treatment. Patients and attendants should be counseled properly regarding the guarded prognosis at the earliest diagnosis of fungal endophthalmitis.
Preparation of antifungals for intravitreal injection
Amphotericin B (5ug/0.1ml)
- Add 10 ml of distilled water in a vial of 50mg amphotericin B and mix well (5 mg/ml)
- Take 0.1 ml of drug solution
- Dilute with 0.9 ml of sterile water and mix well (moving air bubble up and down)
- Discard 0.9 ml
- Again take 0.1 ml of solution and add 0.9 ml distilled water and mix well
- Discard 0.9 ml of solution
- Use 0.1 ml for injection
Voriconazole (50ug/0.1ml)
- Add 19 ml of distilled water in a 200 mg vial of voriconazole and mix well (10 mg/ml)
- Take 0.1 ml solution and add 0.9 ml distilled water and mix well
- Discard 0.5 ml of solution
- Take 0.5 ml of sterile water and mix well (air bubble)
- Discard 0.9 ml of solution
- Use 0.1 ml for injection
Conclusion:
Fungal endophthalmitis requires rapid diagnosis and treatment to save vision. Diagnosis is clinical, supported by a culture of aqueous or vitreous samples and also by blood cultures in patients with endogenous endophthalmitis. PCR can be done for the rapid detection of the fungal pathogen, especially in culture-negative cases. Combined treatment with intravitreal antifungal agents and vitrectomy offers good results.
References:- Gupta A, Gupta V, Gupta A, Dogra MR, Pandav SS, Ray P, Chakraborty A. Spectrum and clinical profile of post cataract surgery endophthalmitis in north India. Indian J Ophthalmol 2003; 51: 139-45.
- Kunimoto DY, Das T, Sharma S, Jalali S, Majji AB, Gopinathan U, Athmanathan S, Rao TN. Microbiologic spectrum and susceptibility of isolates: part I. Postoperative endophthalmitis. Endophthalmitis Research Group. Am J Ophthalmol 1999; 128: 240-2.
- Narang S, Gupta A, Gupta V, Dogra MR, Ram J, Pandav SS, Chakrabarti A. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum, and outcome. Am J Ophthalmol 2001; 132: 609-17.
- Williamson J, Gordon AM, Wood R, Dyer AM, Yahya OA. Fungal flora of the conjunctival sac in health and disease.Influence of topical and systemic steroids. Br J Ophthalmol 1968;52:127-136
- Mitsui Y, Hanabusa J. Corneal infections after cortisone therapy.Br J Ophthalmol. 1955;39(4):244‐250.
- Kresloff MS, Castellarin AA, Zarbin MA. Major review endophthalmitis. Surv Ophthalmol. 1998;43(3):193–224.
- Anand AR, Therese KL, Madhavan HN. Spectrum of aetiological agents of postoperative endophthalmitis and antibiotic susceptibility of bacterial isolates. Indian J Ophthalmol 2000; 48:123–128.
- Chakrabarti A, Shivaprakash MR, Singh R, et al. Fungal endophthalmitis: fourteen years’ experience from a center in India. Retina (Philadelphia, PA). 2008;28:1400–1407.
- Wykoff CC, Flynn HW Jr, Miller D, Scott IU, Alfonso EC. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology 2008; 115: 1501-7, 7.e1-2.
- Pflugfelder SC, Flynn HW Jr, Zwickey TA, Forster RK, Tsiligianni A, Culbertson WW, Mandelbaum S. Exogenous fungal endophthalmitis. Ophthalmology 1988; 95: 19-30.
- Lingappan A, Wykoff CC, Albini TA, Miller D, Pathengay A, Davis JL, Flynn HW Jr. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol 2012; 153: 162-6.e1.
- Schiedler V, Scott IU, Flynn HW Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol 2004; 137: 725-31.
- Essman TF, Flynn HW Jr, Smiddy WE, Brod RD, Murray TG, Davis JL, Rubsamen PE. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers 1997; 28: 185-94.
- Kim DY, Moon HI, Joe SG, et al. Recent clinical manifestation and prognosis of fungal endophthalmitis: a 7-year experience at a tertiary referral center in Korea. J Korean Med Sci.2015;30:960–964.
- Gupta A, Gupta V, Dogra MR, Chakrabarti A,Ray P,Ram J,Patnaik B. Fungal endophthalmitis after a single intravenous administration of presumably contaminated dextrose infusion fluid. Retina. 2000;20:262–268.
- Karkhur S, Afridi R, Menia N, et al. Posterior hypopyon in fungal endogenous endophthalmitis secondary to presumably contaminated dextrose infusion.Am J Ophthalmol Case Rep. 2020;18:100681.
- Ness T1,Pelz K,Hansen LL. Endogenous endophthalmitis: microorganisms, disposition and prognosis. Acta Ophthalmol Scand.2007 Dec;85(8):852-6.
- Ratra D1,Saurabh K,Das D,Nachiappan K,Nagpal A,Rishi E,Bhende P,Sharma T,Gopal L. Endogenous Endophthalmitis: A 10-Year Retrospective Study at a Tertiary Hospital in South India. Asia Pac J Ophthalmol (Phila).2015 Sep-Oct;4(5):286-92.
- Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Survey Ophthalmol . 2003;48:403– 423.
- Gupta S, Loudill C, Tammara A, Chow RT. A rare case of bilateral aspergillus endophthalmitis.J Community Hosp Intern Med Perspect. 2015;5(6):28984.
- Mulla MA,Banker AS,RishiE,Biswas J. Degenerated intravitreal cysticercus cyst masquerading as endogenousendophthalmitis. Ocul Immunol Inflamm.2012;20(5):378-80.
- Maitray A,Rishi E,Rishi P,Gopal L,Bhende P,Ray R,Therese KL. Endogenous endophthalmitis in children and adolescents: Case series and literature review. Indian J Ophthalmol.2019;67(6):795-800.
- RishiP,RishiE,Nandi K,Khan B. Endophthalmitisin eyes presenting with orbital signs: a case-control study. Retina.2010;30(3):491-4.
- Zhang YQ, Wang WJ. Treatment outcomes after pars plana vitrectomy for endogenous endophthalmitis. Retina. 2005;25:746-750.
- Einsele H, Hebart H, Roller G, Loffler J, Rothenhofer I, Muller C A, Bowden R A, van Burik J, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes.J Clin Microbiol.1997;35:1353–1360.
- Miyakawa Y, Mabuchi T, Kahaya K, Fukazawa Y. Isolation and characterization of a species-specific DNA fragment for detection ofCandida albicansby polymerase chain reaction.J Clin Microbiol.1992;30:894–900.
- Durand ML. Bacterial and Fungal Endophthalmitis.Clin Microbiol Rev. 2017;30(3):597‐613.
- Ferrer C, Colom F, Frasés S, Mulet E, Abad JL, Alió JL. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections.J Clin Microbiol. 2001;39(8):2873‐2879.
- Gandhi J, Jayasudha R, Naik P, Sharma S, Dave VP, Joseph J. Targeted High-Throughput Sequencing Identifies Predominantly Fungal Pathogens in Patients with Clinically Infectious, Culture-Negative Endophthalmitis in South India.Microorganisms. 2019;7(10):411.
- Okada AA, Johnson RP, Liles WC, et al. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology. 1994;101:832-838.
- Wong JS, Chan TK, Lee HM, et al. Endogenous bacterial endophthalmitis: an East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000;107:1483-1491.
- Luttrull JK, Leewan W, Kubak BM, Smith MD, Oster HA. Treatment of ocular fungal infections with oral fluconazole. Am J Opthalmol 1995;119:477–481.
- Christmas NJ, Smiddy WE. Vitrectomy and systemic fluconazole for treatment of endogenous fungal endophthalmitis. Ophthal Surg Lasers 1996;27:1012–1018.
- Goodman DF, Stern WH. Oral ketoconazole and intraocular amphotericin for treatment of post-operative Candida parapsilosis endophthalmitis. Arch Ophthalmol 1987;105:172–173.
- Valenton M. Wound infection after cataract surgery. Jpn J Opthalmol 1996;40:447–455.
- Coats MF, Payman JA. Intravitreal corticosteroids in the treatment of fungal endophthalmitis. Retina 1992;12:46–51.
- Stransky TI. Postoperative endophthalmitis secondary to candida parapsilosis. a case treated by vitrectomy and intravitreal antibiotics. Retina 1981;1:179–185.
- Elliot JH, O’Day DM, Gutow GS, Porgorski SF, Akrabawi. Mycotic endophthalmitis in drug abusers. Am J Ophthalmol 1979;88:66–72.
- O’Day DM, Ray WA, Robinson R, Head WS. Efficacy of antifungal agents in the cornea. II. Influence of corticosteroids. Investigative Ophthalmology and Visual Science 1984;25:331–335.
- Maxwell DP, Brent D, Diamond JG, Wu L. Effect of intravitreal dexamethasone on ocular histopathology in a rabbit model of endophthalmitis. Ophthalmology 1991;98:1370–1375.
- Marmor MF. Serous detachment after high dose dexamethasone: toxic or osmotic? Arch Ophthalmol 1993;111:20–21.
- Kwak HW, D’Amico DJ. Evaluation of retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol 1992;110:259–266.
- Diamond RD. Inhibition of monocyte mediated damage to fungal hyphae by steroid hormones. J Infect Dis 1983;174:160.
- Green WR, Bennett JE, Goos RD. Ocular penetration of amphotericin B: a report of laboratory studies and a case report of postsurgical cephalosporium endophthalmitis. Arch Ophthalmol.1965;73:769–775.
- Axelrod AJ, Peyman GA, Apple DJ. Toxicity of intravitreal injection of amphotericin B. Am J Ophthalmol 1973; 76:578–83.
- Krishnan T,RishiP. Management of a case of Candida endogenousendophthalmitisin a neonate. Ocul Immunol Inflamm.2014;22(1):77-8.
- Groll AH, Gullick BM, Petraitiene R, et al. Compartmental pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob Agents Chemother 2001; 45:596–600.
- Groll AH, Mickiene D, Petraitis V, et al. Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin lipopeptide micafungin (FK463) in rabbits. Antimicrob Agents Chemother 2001; 45:3322–7.
- Groll AH, Mickiene D, Petraitiene R, et al. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob Agents Chemother 2001;45:2845–55.
- Riddell J 4th, Comer GM, Kauffman CA. Treatment of endogenous fungal endophthalmitis: focus on new antifungal agents.Clin Infect Dis. 2011;52(5):648‐653.
- Guest JM, Singh PK, Revankar SG, Chandrasekar PH, Kumar A. Isavuconazole for Treatment of Experimental Fungal Endophthalmitis Caused by Aspergillus fumigatus.Antimicrob Agents Chemother. 2018;62(11):e01537-18.
- Sen S, Lalitha P, Mishra C, ParidaH,RameshkumarG,KannanNB,Ramasamy K. Post-cataract Surgery Fungal Endophthalmitis: Management Outcomes and Prognostic Factors [published online ahead of print, 2020 Apr 10].Ocul Immunol Inflamm. 2020;1‐7.
- Ozdamar A, Aras C, Ozturk R, Akin E, Karacorlu M, Ercikan C. In vitro antimicrobial activity of silicone oil against endophthalmitis-causing agents. Retina 1999;19(2):122-126.
- Azad R, Ravi K, Talwar D, Rajpal, Kumar N. Pars plana vitrectomy with or without silicone oil endotamponade in post-traumatic endophthalmitis. Graefes Arch Clin Exp Ophthalmol 2003;241(6):478-483.
- Ornek N, Apan T, O urel R, Ornek K. Comparison of the antimicrobial effect of heavy silicone oil and conventional silicone oil against endophthalmitis-causing agents. Indian J ophthalmol 2014;62(4):388-391.
- Hegazy HM, Kivilcim M, Peyman GA, Unal MH, Liang C, Molinari LC, Kazi AA. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir in a silicone oil-filled eye. Retina 1999;19(6):553-557.
- Vinekar A, Dogra MR, Avadhani K, Gupta V, Gupta A, Chakrabarti A. Management of recurrent postoperative fungal endophthalmitis. Indian J Ophthalmol 2014;62:136-40.

