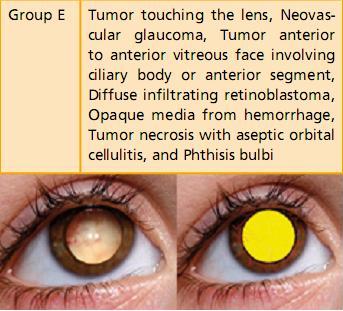
Introduction
Retinoblastoma is the most common intraocular malignancy in children, with a reported incidence ranging from 1 in 15,000 to 1 in 18,000 live births.1 It is second only to uveal melanoma in the frequency of occurrence of malignant intraocular tumors. There is no racial or gender predisposition in the incidence of retinoblastoma. Retinoblastoma is bilateral in about 25 to 35% of cases. 2 The average age at diagnosis is 18 months, unilateral cases being diagnosed at around 24 months and bilat eral cases before 12 months. 2
Pawius described retinoblastoma as early as in 1597.3 In 1809, Wardrop re ferred to the tumor as fungus haematodes and suggested enucleation as the primary mode of management.3 The discovery of ophthalmoloscope in 1851 facilitated recognition of specific clini cal features of retinoblastoma. Initially thought to be derived from the glial cells, it was called a glioma of the ret ina by Virchow (1864)3.Flexner (1891) and Wintersteiner (1897) believed it to be a neuroepithelioma because of the presence of rosettes.3Later, there was a consensus that the tumor originated from the retinoblasts and the Ameri can Ophthalmological Society officially accepted the term retinoblastoma in 1926.4
Retinoblastoma was associated with near certain death just over a century ago. Early tumor recognition aided by indirect ophthalmoscopy and refined enucleation technique contributed to an improved survival from 5% in 1896 to 81% in 1967. 2 Advances in external beam radiotherapy in the 1960s and 1970s and further progress in planning and delivery provided an excellent alternative to enucleation and resulted in substantial eye salvage.2 Focal therapeutic measures such as cryotherapy, photocoagulation and plaque brachy therapy allowed targeted treatment of smaller tumors entailing vision salvage. Parallel advancements in ophthalmic diagnostics and introduction of ultra sonography, computed tomography, and magnetic resonance imaging con tributed to improved diagnostic accu racy and early detection of extraocular retinoblastoma.
Despite all the advances that took place between 1960 and 1990, the overall management of retinoblastoma stood at cross roads in the 1990s. The outstanding issues related related to identif cation of a child at risk of developing retinoblastoma by genetic testing, op timization of vision salvage by minimi zation of the size of the tumor regres sion scar, reduction in the incidence of second malignant neoplasm following external beam radiotherapy by explor ing for alternative therapeutic modalities, reduction in the incidence of sys temic metastasis following enucleation, and improvement in the prognosis of orbital retinoblastoma and metastatic retinoblastoma.
The recent advances such as identific tion of genetic mutations,5,6 replace ment of external beam radiotherapy by chemoreduction as the primary man agement modality, use of chemoreduc tion to minimize the size of regression scar with consequent optimization of visual potential,7-11identification of histopathologic high-risk factors follow ing enucleation 12 and provision of adjuvant therapy to reduce the incidence of systemic metastasis,13 protocol-based management of retinoblastoma with accidental perforation or intraocular surgery14-16 and aggressive multimodal therapy in the management of orbital retinoblastoma17,18 have contributed to improved outcome in terms of better survival, improved eye salvage and potential for optimal visual recovery.
Genetics of Retinoblastoma
Out of the newly diagnosed cases of retinoblastoma only 6% are familial while 94% are sporadic.2,19 Bilateral retinoblastomas involve germinal mutations in all cases. Approximately 15% of unilateral sporadic retinoblastoma is caused by germinal mutations affecting only one eye while the 85% are sporadic.2
In 1971, Knudson proposed the two hit hypothesis. He stated that for retino blastoma to develop, two chromosom al mutations are needed.20 In hereditary retinoblastoma, the initial hit is a germi nal mutation, which is inherited and is found in all the cells. The second hit de velops in the somatic retinal cells lead ing to the development of retinoblastoma. Therefore, hereditary cases are predisposed to the development of no nocular tumors such as osteosarcoma.
In unilateral sporadic retinoblastoma, both the hits occur during the devel opment of the retina and are somatic mutations. Therefore there is no risk of second nonocular tumors.
Genetic counseling is an important aspect in the management of retinoblastoma. In patients with a positive family history, 40% of the siblings would be at risk of developing retinoblastoma and 40% of the offspring of the affected patient may develop retinoblas toma. In patients with no family history of retinoblastoma, if the affected child has unilateral retinoblastoma, 1% of the siblings are at risk and 8% of the offspring may develop retinoblas toma. In cases of bilateral retinoblastoma with no positive family history, 6% of the siblings and 40% of the offspring have a chance of developing retinoblastoma.2
Apart from empiric genetic counseling as described above, the current trend is to identify the mutation and compute specifc antenatal risk.We screened twenty-one probands, twelve with bilateral retinoblastoma and 9 with unilateral retinoblastoma, for mutations in the RB1 gene using genomic DNA from peripheral blood leukocytes as well as tumors. Amplifcation of individual exons and fanking regions of the RB1 gene were carried out, followed by direct sequencing of the amplifed products. Sequences of affected individuals were compared with those of controls. Mutations were identifed in seven patients, fve with bilateral and two with unilateral retinoblastoma. Analysis of the peripheral blood of seven patients with unilateral disease showed no mutations. 5
Subsequently, we carried out mutational screening of the exons and promoter of the RB1 gene in Indian patients with retinoblastoma in order to determine the range of mutations giving rise to the disease. Eight novel mutations were identified, including 4 single base changes, 2 small deletions and 1 duplication. These were g.64365T>G (Tyr325Ter), g.78131G>A (Trp515Ter), g.150061G>T (Glu 587Ter), g.170383C>G (S834X), g.41924A>C (IVS3-2A>C),g.150064ins4, g.160792del22, and g.76940del14 (IVS15 del +20-33). All mutations pro duced nonsense codons or frameshifts. Detectable mutations in exons were found in 46% of patients tested.6Knowledge of the full range of muta tions can aid in the design of screening tests for individuals at risk.6
Histopathology of Retinoblastoma
On low magnification, basophilic areas of tumor are seen along with eosinophilic areas of necrosis and more basophilic areas of calcification within the tumor. Poorly differentiated tumors consist of small to medium sized round cells with large hyperchromatic nuclei and scanty cytoplasm with mitotic figures.Well-dif ferentiated tumors show the presence of rosettes and fleurettes.These can be of various types. Flexner-Wintersteiner rosettes consist of columnar cells ar ranged around a central lumen. This is highly characteristic of retinoblastoma and is also seen in medulloepithelioma. Homer Wright rosettes consist of cells arranged around a central neuromuscular tangle. This is also found in neu roblastomas, medulloblastomas and medulloepitheliomas. Pseudorosette refers to the arrangement of tumor cells around blood vessels. They are not signs of good differentiation. Fleurettes are eosinophilic structures composed of tumor cells with pear shaped eosinophilic processes projecting through a fenestrated membrane. Rosettes and fleurettes indicate that the tumor cells show photoreceptor differentiation. In addition basophilic deposits (precipitated DNA released after tumor necrosis) can be found in the walls of the lumen of blood vessels.2
Clinical Manifestations of Retinoblastoma
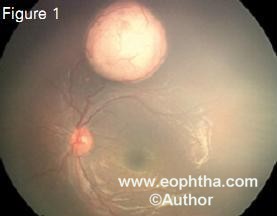
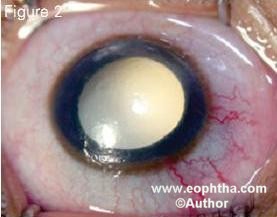
Leucocoria is the most common presenting feature of retinoblastoma, fol lowed by strabismus, painful blind eye and loss of vision. Table 1 lists the common presenting signs and symptoms of retinoblastoma 21.The clinical presentation of retinoblas toma depends on the stage of the disease 10.Early lesions are likely to be missed, unless an indirect ophthalmos copy is performed. The tumor appears as a translucent or white fluffy retinal mass (Figure 1). The child may present with strabismus if the tumor involves the macula or with reduced visual acu ity. 10 Moderately advanced lesions usually present with leucocoria due to the re flection of light by the white mass in the fundus (Figure 2).
Figure 1. Early manifestation of retinoblastoma with a localized tumor
Figure 2. Lecocoria is the most common clinical presentation of retinoblastoma
As the tumor grows further, three patterns are usually seen:
| Leucocoria | 56% |
| Strabismus | 20% |
| Red painful eye | 7% |
| Poor vision | 5% |
| Asymptomatic | 3% |
| Orbital Cellulitis | 3% |
| Unilateral Mydriasis | 2% |
| Heterochromia Iridis | 1% |
| Hyphema | 1% |
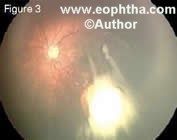
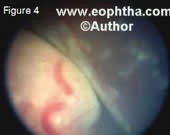
Endophytic, in which the tumor grows into the vitreous cavity (Figure 3). A yellow white mass progressively fills the entire vitreous cavity and vitreous seeds occur. The retinal vessels are not seen on the tumor surface.Exophytic, in which the tumor grows towards the subretinal space (Figure 4). Retinal detachment usually occurs and retinal vessels are seen over the tumor.Diffuse infiltrating tumor, in which the tumor diffusely involves the retina causing just a placoid thickness of the retina and not a mass. This is gener ally seen in older children and usually there is a delay in the diagnosis (Figure 5).
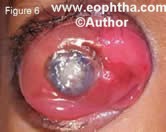
Advanced tumors manifest with prop tosis secondary to optic nerve extension or orbital extension (Figure 6) and systemic metastasis.10 Retinoblastoma can spread through the optic nerve with relative ease especially once the lamina cribrosa is breached. Orbital extension may present with proptosis and is most likely to occur at the site of the scleral emissary veins. Systemic metastasis oc curs to the brain, skull, distant bones and the lymph nodes.
Figure 3. Endophytic tumor with vitreous seeds
Figure 4. Exophytic retinal tumor with exuda tive retinal detachment
Figure 5. Diffuse infiltrative retinoblastoma with placoid retinal thickening seen on gross examination of the enucleated eye in a 7-year old child
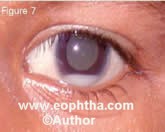
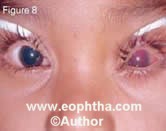
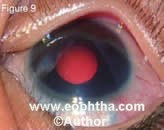
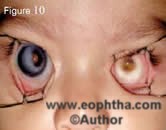
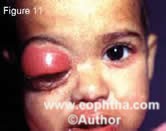
Some of the atypical manifestations of retinoblastoma include pseudohypopy on (Figure 7), spontaneous hyphema (Figure 8), vitreous hemorrhage (Figure 9), phthisis bulbi (Figure 10) and preseptal or orbital cellulites (Figure 11).
Figure 6. Retinoblastoma with orbital exten sion in a 3-year-old child
Figure 7. A 5-year-old child with retinoblasto ma with anterior segment seeding manifesting with tumor hypopyon
Figure 8. A 4-year-old with spontaneous hy phema in the left eye. Ultrasonography con firmed the diagnosis of retinoblastoma
Figure 9. Spontaneous vitreous hemorrhage as the presenting feature of retinoblastoma in a 4-year-old child
Figure 10. An 18-month-old child with bilateral retinoblastoma. The right eye has secondary glaucoma and enlarged cornea while the left eye is phthisical.
Figure 11. A 3-year-old child with retinoblas toma presenting with orbital cellulites
Diagnosis of Retinoblastoma
A thorough clinical evaluation with careful attention to details, aided by ultrasonography B-scan helps in the diagnosis.10 Computed tomography and magnetic resonance imaging are generally reserved for cases with atypical manifestations and diagnostic dilemma and where extraocular or intracranial tumor extension is suspected. 10
A child with suspected retinoblastoma necessarily needs complete ophthalmic evaluation including a dilated fundus examination under anaesthesia.10 The intraocular pressure is measured and the anterior segment is examined for neovascularization, pseudohypopyon, hyphema, and signs of inflammation 10
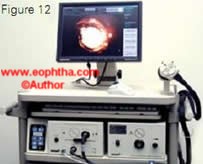
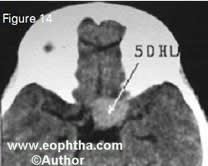
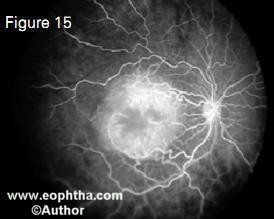
Bilateral fundus examination with 360 degree scleral depression is mandatory. Direct visualization of the tumor by an indirect ophthalmoscope is diagnostic of retinoblastoma in over 90% of cases.21 RetCam is a wide-angle fundus camera, useful in accurately document ing retinoblastoma and monitoring re sponse to therapy (Figure 12).Ultrasonography B-scan shows a rounded or irregular intraocular mass with high internal reflectivity representing typical intralesional calcification (Figure 13). 10 Computed tomography delineates extraocular extension and can detect an associated pinealoblastoma (Figure 14).10 Magnetic resonance imaging is specifically indicated if optic nerve invasion or intracranial extension is suspected. 10On fluorescein angiography, smaller retinoblastoma shows min imally dilated feeding vessels in the arterial phase, blotchy hyperfluorescence in the venous phase and late staining (Figure 15).10
Figure 12. RetCam, a wide-angle digital fun dus camera and image archival system helps in documentation and assessment of tumor regression on follow-up
Figure 13. Ultrasonography B-scan showing multifocal retinal tumors
Figure 14. Computed tomography scan shows pinealoblastoma
Figure 15. Fundus fluorescein angiography in retinoblastoma in the early phase shows blotchy hyperfluorescence
Classification of Retinoblastoma
An ideal classificationsystem for retinoblastoma should include two components: grouping and staging. Grouping is a clinical system of prognosticating organ salvage while staging prognosti cates survival.22
The Reese Ellsworth classification was introduced to prognosticate patients treated with methods other than enucleation. This classification was devised prior to the widespread use of indirect ophthalmoscopy and focal measures of management of retino blastoma and mainly pertained to eye salvage with external beam radiotherapy. Although the Essen classification addressed some of the shortcomings of Reese Ellsworth classification, it is considered too complex.
Further, none of the older systems of classification had been designed to prognosticate chemoreduction, the current favored method of retinoblastoma management. The new International Classification of Intraocular Retinoblastoma is a logical flow of sequential tumor grading that linearly correlates with the outcome of newer therapeutic modalities (Table 2).23, 24
Table 2:International Classification of Intraocular RetinoblastomaCourtesy Carol L Shields, MD, Wills Eye Institute, Philadelphia, PA, USA
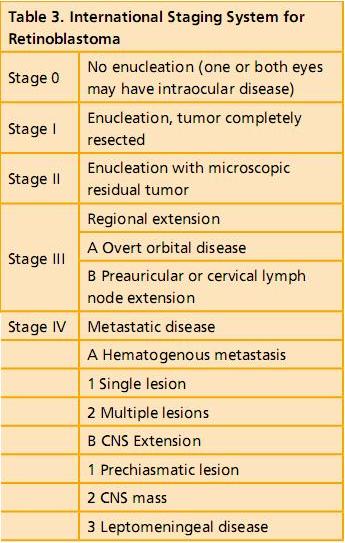
The new International Staging system is the first such for retinoblastoma and incorporates five distinct stages (Table 3).25 Staging is based on collective information gathered by the clinical evaluation, imaging, systemic survey and histopathology.
Management of Retinoblastoma
The primary goal of management of retinoblastoma is to save life. Salvage of the organ (eye) and function (vision) are the secondary and tertiary goals respectively. The management of retin oblastoma needs a multidisciplinary team approach including an ocular on cologist, pediatric oncologist, radiation oncologist, radiation physicist, genetist and an ophthalmic oncopathologist. The management strategy depends on the stage of the disease – intraocular retinoblastoma, retinoblastoma with high-risk characteristics, orbital retino blastoma and metastatic retinoblas toma.
Management of retinoblastoma is highly individualized and is based on several considerations - age at presentation, laterality, tumor location, tumor stag ing, visual prognosis, systemic condition, family and societal perception, and, to a certain extent, the overall prognosis and cost-effectiveness of treatment in a given economic situation .
Management of Intraocular Retinoblastoma
A majority of children with retinoblas toma manifest at the stage when the tumor is confined to the eye. About 90-95% of children in developed countries present with intraocular retinoblasto ma while 60-70% present at this stage in the developing world.10 Diagnosis of retinoblastoma at this stage and appro priate management are crucial for life, eye and possible vision salvage.
There are several methods to manage intraocular retinoblastoma - focal (cryotherapy, laser photocoagulation, transpupillary thermotherapy, transcle ral thermotherapy, plaque brachytherapy), local (external beam radiotherapy, enucleation), and systemic (chemother apy). While primary focal measures are mainly reserved for small tumors, local and systemic modalities are used to treat advanced retinoblastoma.
Cryotherapy
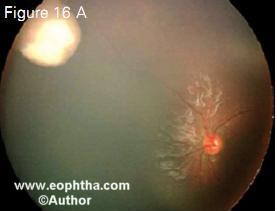
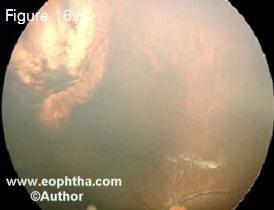
Cryotherapy is performed for small equatorial and peripheral retinal tumors measuring up to 4 mm in basal diameter and 2 mm in thickness.2,10 Triple freeze thaw cryotherapy is applied at 4-6 week intervals until complete tumor regression. Cryotherapy produces a scar much larger than the tumor (Figure 16). Complications of cryotherapy include transient serous retinal detachment, retinal tear and rhegmatogenous reti nal detachment. Cryotherapy administered 2-3 hours prior to chemotherapy can increase the delivery of chemo therapeutic agents across the blood retinal barrier and thus has synergistic effect.10
Figure 16. A peripheral retinal tumor that underwent cryotherapy (16 A). The tumor has completely regressed but the scar is much larger than the tumor itself (16 B).
Laser Photocoagulation
Laser photcoagulation is used for small posterior tumors 4 mm in basal diame ter and 2 mm in thickness.2,10 The treatment is directed to delimit the tumor and coagulate the blood supply to the tumor by surrounding it with two rows of overlapping laser burns. Complica tions include transient serous retinal detachment, retinal vascular occlusion, retinal hole, retinal traction, and pre retinal fibrosis. It is less often employed now with the advent of thermotherapy. In fact, laser photocoagulation is contraindicated while the patient is on active chemoreduction protocol.10
Thermotherapy
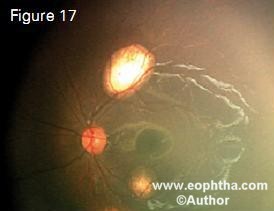
In thermotherapy, focused heat generated by infrared radiation is applied to tissues at subphotocoagulation levels to induce tumor necrosis.26 The goal is to achieve a slow and sustained temperature range of 40 to 60 degree C within the tumor, thus sparing damage to the retinal vessels (Figure 17). Transpupil lary thermotherapy using infrared ra diation from a semiconductor diode laser delivered with a 1300-micron large spot indirect ophthalmoscope delivery system has become a standard practice. It can also be applied transpupil lary through an operating microscope or by the transscleral route with a di opexy probe. The tumor is heated until it turns a subtle gray. Thermotherapy provides satisfactory control for small tumors - 4 mm in basal diameter and 2 mm in thickness. Complete tumor regression can be achieved in over 85% of tumors using 3-4 sessions of thermo therapy.26The common complications are focal iris atrophy, focal paraxial lens opacity, retinal traction and serous retinal detachment. The major applica tion of thermotherapy is as an adjunct to chemoreduction. The application of heat amplifies the cytotoxic effect of platinum analogues. This synergistic combination with chemoreduction pro tocol is termed chemothermotherapy.
Figure 17. Two focal tumors treated with transpupillary thermotherapy: note flat scars with patent blood vessels coursing through the scars. Transpupillary thermotherapy classically spares the blood vessels from occlusion and produces a compact scar
Plaque Brachytherapy
Plaque brachytherapy involves placement of a radioactive implant on the sclera corresponding to the base of the tumor to transsclerally irradiate the tumor.27 Commonly used radioactive materials include Ruthenium 106 (Figure 18) and Iodine 125. The advantages of plaque brachytherapy are focal deliv ery of radiation with minimal damage to the surrounding normal structures, minimal periorbital tissue damage, ab sence of cosmetic abnormality because of retarded bone growth in the field of irradiation as occurs with external beam radiotherapy, reduced risk of sec ond malignant neoplasm and shorter duration of treatment.
Figure 18. Ruthenium 106 plaque
Plaque brachytherapy is indicated in tumors less than 16 mm in basal diameter and less than 8 mm thickness. It could be the primary or secondary mo dality of management. Primary plaque brachytherapy is currently performed only in situations where chemotherapy is contraindicated. It is most useful as secondary treatment in eyes that fail to respond to chemoreduction and external beam radiotherapy or for tumor recurrences.
Plaque brachytherapy requires precise tumor localization and measurement of its basal dimensions. The tumor thickness is measured by ultrasonog raphy. The data is used for dosimetry on a three-dimensional computerized tumor modeling system. The plaque design is chosen depending on the ba sal tumor dimensions, its location, and configuration. The dose to the tumor apex ranges from 4000-5000 cGy. The plaque is sutured to the sclera after confirming tumor centration and is left in situ for the duration of exposure, generally ranging from 36 to 72 hours. The results of plaque brachytherapy are gratifying with about 90% tumor control. The common complications are radiation papillopathy and radiation retinopathy.
External Beam Radiotherapy
External beam radiotherapy was the preferred form of management of mod erately advanced retinoblastoma in late 1900s.28, 29 However with the advent of newer chemotherapy protocols, external beam radiotherapy is being used less often. Presently it is indicated in eyes where primary chemotherapy and local therapy has failed, or rarely when chemotherapy is contraindicated. 10
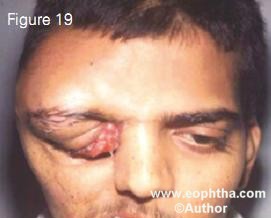
The major problems with external beam radiotherapy are the stunting of the orbital growth, dry eye, cataract, radiation retinopathy and optic neuropathy. External beam radiotherapy can induce second malignant neoplasm especially in patients with the hereditary form of retinoblastoma (Figure 19). There is a high 30% chance of developing another malignancy by the age of 30 years in such patients if they are given external beam radiotherapy compared to a less than 6% chance in those who do not receive external beam radiotherapy.30 The risk of second malignant neoplasm is greater in children under 12 months of age. 30
Figure 19. Osteosarcoma of the frontal bone in a 20-year-old patient with bilateral retino blastoma who had undergone external beam radiotherapy at 1-year age
Enucleation
Enucleation is a common method of managing advanced retinoblastoma. Just about 3 decades ago,a majority of patients with unilateral retinoblastoma and the worse eye in bilateral retinoblastoma underwent primary enuclea tion. A substantial reduction in the frequency of enucleation has occurred in the late last century.31 Concurrently, there has been an increase in the use of alternative eye- and vision-conserving methods of treatment.9, 32
Primary enucleation continues to be the treatment of choice for advanced intraocular retinoblastoma with neovascularization of iris, secondary glau coma, anterior chamber tumor invasion, tumors occupying >75% of the vitreous volume, necrotic tumors with second ary orbital inflammation, and tumors associated with hyphema or vitreous hemorrhage where the tumor characteristics can not be visualized, especially when only one eye is involved. 10
| a. | Minimal manipulation |
| b. |
Avoid perforation of the eye |
| c. |
Harvest long (> 15 mm) optic nerve stump |
| d. |
Inspect the enucleated eye for macroscopic extraocular extension and optic nerve involvement |
| e. |
Harvest fresh tissue for genetic studies |
| f. |
Avoid biointegrated implant if postoperative radiotherapy is necessary |
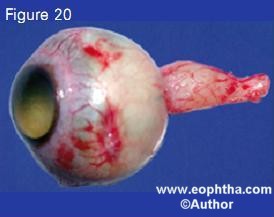
There are specific considerations while enucleating an eye with retinoblastoma. (Table 5) Minimum-manipulation surgical technique should be necessarily practiced 11.It is important not to accidentally perforate the eye. The sclera is thin at the site of muscle insertions and the rectus muscles have to be hooked delicately. It is important to obtain a long optic nerve stump, ideally more than 15 mm, but never less than 10 mm (Figure 20). 11
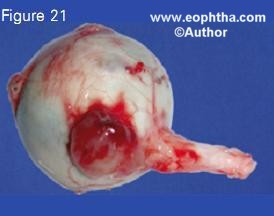
Certain steps can be taken to obtain about 15 mm long optic nerve stump in all cases of advanced retinoblastoma.11 Gentle traction can be applied by the traction sutures applied to recti muscle stumps prior to transecting the optic nerve. As an alternative to the traction sutures, medial or lateral rectus muscle stumps may be kept long and traction exerted with an artery clamp. A 15-degree curved and blunt-tipped tenotomy scissors is introduced from the lateral aspect (or a straight scissors from the medial aspect) and the optic nerve is palpated with the closed tip of the scissors while maintaining gentle traction on the eyeball. The scissors is moved posteriorly to touch the orbital apex while “strumming” the optic nerve. The scissors is lifted by 3 or 4 millimeters off the orbital apex (to preserve the con- tents of the superior orbital fissure), the blades of the scissors are opened to engage the optic nerve, and the nerve is transected with one bold cut. This maneuver generally provides at least 15 mm long optic nerve stump. 11 Enucleation spoon and heavy enucleation scissors limit space for maneuverability and may result in a shorter optic nerve stump. In addition, one should be careful not to accidentally perforate the eye during enucleation. The enucleated eyeball is inspected for optic nerve (Figure 20) or extraocular extension (Figure 21) of tumor.
Figure 20. Enucleated eyeball showing 18 mm optic nerve stump. Note the proximal portion of the optic nerve is thickened indicating tumor infiltration
Figure 21. Enucleated eyeball showing extras cleral tumor extension
Eyes manifesting tumor necrosis with aseptic orbital cellulitis pose specific problem. These patients should be im aged to rule out extraocular extension. Enucleation is best performed when the inflammation is resolved.11 A brief course of preoperative oral and topical steroids help control inflammation. Patients with retinoblastoma present ing as phthisis bulbi need imaging to exclude extraocular and optic nerve extension. 11 Phthisis generally results following spontaneous tumor necrosis and an episode of aseptic intraocular and orbital inflammation. Enucleation in these cases is often complicated by excessive peribulbar fibrosis and intra operative bleeding. 11
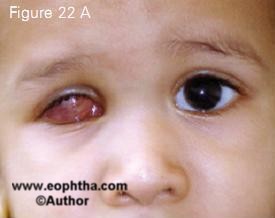
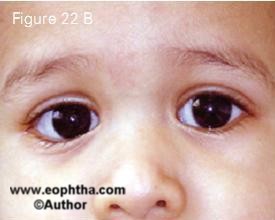
Placement of an orbital implant following enucleation for retinoblastoma is the current standard of care. The orbital implant promotes orbital growth, provides better cosmesis and enhances prosthesis motility. The implants could be non-integrated (polymethyl methacrylate or silicon) or bio-integrated (hydroxyapatite or porous polyethylene). Placement of a biointegrated implant is generally avoided if post-operative adjuvant radiotherapy is considered necessary 11. Although most implants structurally tolerate radiotherapy well, implant vascularization may be com promised by radiotherapy thus increas ing the risk of implant exposure. Use of myoconjunctival technique and custom ocular prosthesis have optimized pros thesis motility and static cosmesis (Figure 22).
Figure 22. Retinoblastoma in the right eye following enucleation with orbital implant by the myoconjunctival technique (22 A). Excellent cosmesis follwing fitting of a custom ocular prosthesis (22 B).
Chemotherapy
Chemoreduction, defned as the process of reduction in the tumor volume with chemotherapy, has become an integral part of the current management of retinoblastoma 32.Chemotherapy alone is however not curative and must be associated with intensive local therapy. Chemoreduction coupled with focal therapy can minimize the need for enucleation or external beam radiotherapy without signifcant systemic toxicity.
Chemoreduction in combination with focal therapy is now extensively used in the primary management of retinoblastoma.33-36There are different protocols in chemotherapy. The commonly used drugs are vincristine, etoposide and carboplatin, for 6 cycles. 7-10 (Table 6) Standard dose chemoreduction is provided in ICIOR groups A-C.10 In high dose chemoreduction, the dose of etoposide and carboplatin is increased. This is indicated in ICIOR groups D tumors 10
| Day 1:Vincristine + Etoposide + Carboplatin |
| Day 2:Etoposide |
|
Standard dose(3 weekly, 6 cycles): Vincristine 1.5 mg/m22 (0.05 mg/kg for children < 36 months of age and maximum dose < 2mg), Etoposide 150 mg/m (5 mg/kg for children < 36 months of age), Carboplatin 560 mg/m2 (18.6 mg/kg for children < 36 months of age) |
| High-dose(3 weekly, 6-12 cycles): Vincristine 0.025 mg/Kg, Etoposide 12 mg/Kg, Carboplatin 28 mg/Kg |
With chemoreduction and sequential local therapy, it is now possible to salvage many an eye and maximize residual vision. Chemoreduction is most successful for tumors without associated subretinal fuid or vitreous seeding 7,8. Risk factors for tumor, subretinal seed and vitreous seed recurrence, and failure of chemoreduction leading to external beam radiotherapy and/or enucleation have been identifed. 7,8 Chemoreduction offers satisfactory tumor control for Reese Ellsworth groups I-IV eyes, with treatment failure necessitating additional external beam radiotherapy in only 10% and enucleation in 15% at 5-year followup. Patients with Reese Ellsworth group V eyes require external beam radiotherapy in 47% and enucleation in 53% at 5 years.7,8 Chemoreduction is an option for selected eyes with unilateral retinoblastoma. 9
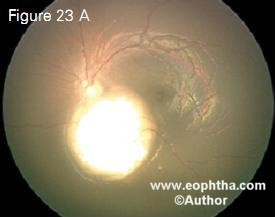
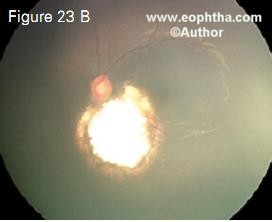
Figure 23. Juxtapapillary retinoblastoma in a 6month-old child (23 A), completely regressed with 6 cycles of chemoreduction alone (23 B)
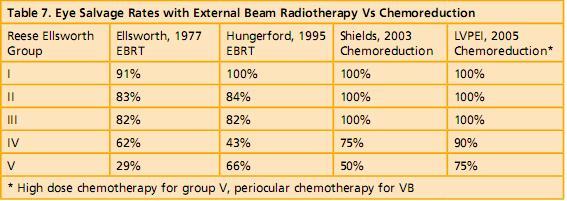
Figure 23 shows a juxtapapillary tumor regressed with chemoreduction alone. Transpupillary thermotherapy was not performed because of the crucial location. Figure 24, 25 and 26 show that the resulting scar with chemoreduction was much smaller than the original tumor with the foveola fully exposed, thus maximizing visual potential. With the modifed protocol that we use specifcally for advanced retinoblastoma, our eye salvage rates are 100% for Reese Ellsworth groups 1-3, 90% for group D and 75% for group E (Table 7).
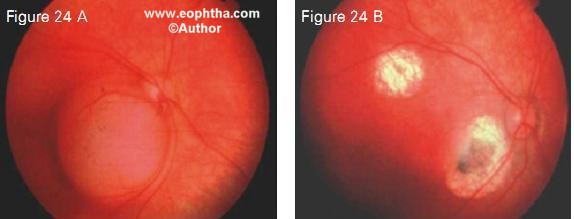
Figure 24. Multifocal retinoblastoma (24 A) following chemoreduction and transpupillary thermotherapy (24 B). Note flat scars that are much smaller than the original tumor.
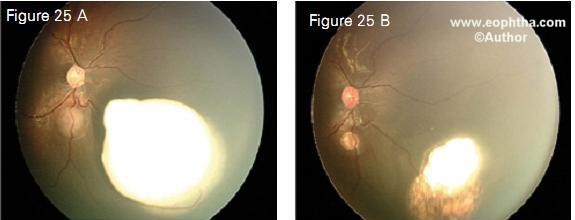
Figure 25. Multifocal retinoblastoma (25 A) regressed following chemoreduction and transpupillary thermotherapy (25 B)
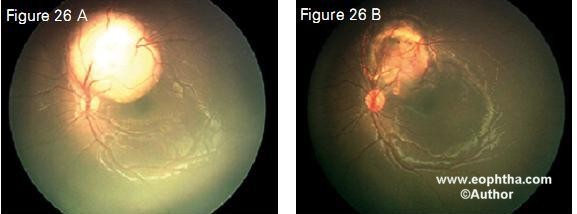
Figure 26. A juxtapapillary retinal tumor in a 9-month-old child (26 A) completely regressed with 6 cycles of chemoreduction and transpupillary thermotherapy (26 B). Note the completely exposed fovea following treatment, thus maximizing visual potential.
It is important to be aware of the adverse effects and interactions of chemotherapeutic agents, which include myelosuppression, febrile episodes, neurotoxicity and non-specifc gastrointestinal toxicity. Chemotherapy should be given only under the supervision of an experienced pediatric oncologist.
Periocular Chemotherapy
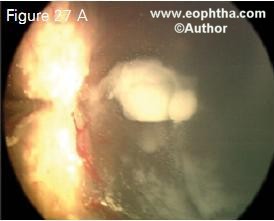
Carboplatin delivered deep posterior subtenon has been demonstrated to be efficacious in the management of Reese Ellsworth Group VB retinoblas toma with vitreous seeds because it can penetrate the sclera and achieve effective concentrations in the vitreous cavity. This modality is currently under trial. Our early results have shown that periocular chemotherapy achieves 70% eye salvage in patients with retinoblastoma with diffuse vitreous seeds (Figure 27).37
Figure 27. Retinoblastoma with massive vitreous seeds (Figure 27 A). Following 6 cycles of high-dose chemoreduction and periocular carboplatin injection, the tumour and the vitreous seeds show complete regression (Figure 27 B).
Follow-up Schedule
The usual protocol is to schedule the first examination 3–6 weeks after the initial therapy. In cases where chemoreduction therapy has been administered, the examination should be done every 3 weeks with each cycle of chemother apy. Patients under focal therapy are evaluated and treated every 4-8 weeks until complete tumor regression. Fol lowing tumor regression, subsequent examination should be 3 monthly for the first year, 6 monthly for three years or until the child attains 6 years of age, and yearly thereafter.
High Risk Retinoblastoma
Systemic metastasis is the main cause for mortality in patients with retino blastoma. Although the life prognosis of patients with retinoblastoma has dramatically improved in the last three decades, with a reported survival of more than 90% in developed countries,38 mortality is still as high as 50% in the developing nations. 39,40 Reduction in the rate of systemic metastasis by identification of high-risk factors and appropriate adjuvant therapy may help improve survival.
High–Risk Factors
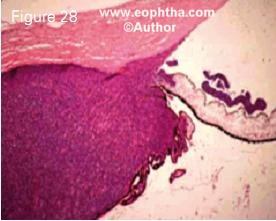
Figure 28. Histopathology of retinoblastoma showing anterior chamber seeding, iris infiltration, trabecular meshwork infiltration and cili ary body invasion
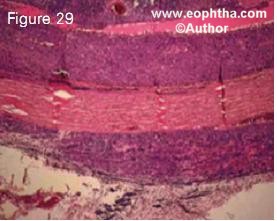
Figure 29. Histopathology of retinoblastoma showing massive choroidal infiltration, scleral infiltration and extrascleral extension
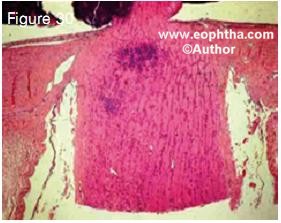
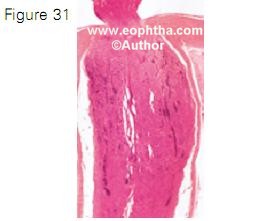
Figure 30. Histopathology of retinoblastoma showing infiltration of the optic nerve beyond the lamina cribrosa
Figure 31. Histopathology of retinoblastoma showing optic nerve infiltration to the level of transection
None of the clinical high-risk factors seem to strongly correlate with mortality. Recent studies have evaluated the role of histopathologic high-risk factors identified following enucleation. The identification of frequency and signifi cance of high-risk histopathologic factors (Figures 28-31) that can reliably predict metastasis is vital for patient selection for adjuvant therapy. It is now generally agreed that massive choroidal infiltration, retrolaminar op tic nerve invasion, invasion of the optic nerve to transection, scleral infiltration, and extrascleral extension are the risk factors that are predictive of metastasis (Table 8). 39, 41-49
| 1 | Anterior chamber seeding |
| 2 | Iris infiltration |
| 3 | Ciliary body infiltration |
| 4 | Massive choroidal infiltration |
| 5 | Invasion of the optic nerve lamina cribrosa |
| 6 | Retrolaminar optic nerve invasion |
| 7 | Invasion of optic nerve transection |
| 8 | Scleral infiltration |
| 9 | Extrascleral extension |
The reported occurrence of ante rior chamber seeding (7%)45,massive choroidal infiltration (12-23%)43-49 ,invasion of optic nerve lamina cribrosa (6-7%)43-49, retrolaminar optic nerve invasion (6-12%), invasion of optic nerve transection (1-25%)43-49 ,scleral infiltration (1-8%)43-49, and extrascleral extension (2-13%)43-49, widely vary even in developed countries. Vemuganti and associates have reported that 21% of the 76 eyes enucleated for advanced retinoblastoma in India had anterior chamber seeding, 54% had massive choroidal infiltration, 46% had optic nerve invasion at or beyond the lamina cribrosa and 7% had scleral infiltration or extrascleral extension.12 It is apparent that the incidence of histopatho logic risk factors is strikingly high in developing countries compared to the published data from developed coun tries.
Adjuvant Therapy
Studies on the efficacy of adjuvant therapy to minimize the risk of metas tasis initiated in the 1970s were marked by variable results and provided no firm recommendation.18 A recent study with a long-term follow-up provides useful information.13, 50 It included a subset of patients with unilateral sporadic retinoblastoma who underwent primary enucleation. The study used specific predetermined histopathologic charac teristics for patient selection. A mini mum follow-up of 1 year was allowed to include metastatic events that generally occur at a mean of 9 months following enucleation.13,50The incidence of metastasis was 4% in those who received adjuvant therapy compared to 24% in those who did not. The study found that administration of adjuvant therapy significantly reduced the risk of metastasis in patients with high-risk histopathologic characteristics.
Our current practice is to administer 6 cycles of a combination of carbopla tin, etoposide and vincristine (identical to the protocol used for chemoreduction of intraocular retinoblastoma) in patients with histopathologic high-risk characteristics. All patients with exten sion of retinoblastoma up to the level of optic nerve transection, scleral infiltra tion, and extrascleral extension receive high dose chemotherapy for 12 cycles and fractionated 4500 to 5000 cGy or bital external beam radiotherapy.
Conclusion
There has been a dramatic change in the overall management of retinoblast oma in the last decade. Specific genetic protocols have been able to make pre natal diagnosis of retinoblastoma. Early diagnosis and advancements in focal therapy have resulted in improved eye and vision salvage. Chemoreduction has become the standard of care for the management of moderately advanced intraocular retinoblastoma. Periocular chemotherapy is now an additional use ful tool in salvaging eyes with vitreous seeds. Enucleation continues to be the preferred primary treatment approach in unilateral advanced retinoblastoma. Post-enucelation protocol, including identification of histopathologic highrisk characteristics and provision of adjuvant therapy has resulted in sub stantial reduction in the incidence of systemic metastasis. The vexing orbital retinoblastoma now seems to have a cure finally with the aggressive multi modal approach. Future holds promise for further advancement in focal thera py and targeted drug delivery.
References:
1. Bishop JO, Madsen EC: Retinoblas toma. Review of current status. Surv Ophthalmol 19: 342-366, 1975.
2. Shields JA, Shields CL. Intraocular tumors – A text and Atlas. Philadel phia, PA, USA, WB Saunders Company, 1992.
3. Albert DM: Historic review of retinoblastoma. Ophthalmology 94; 654-662, 1987.
4. Jackson E: Report of the committee to investigate and revise the classifica tion of certain retinal conditions. Trans Am Ophthalmol Soc 24:38-39, 1926
5. Ata-ur-Rasheed M, Vemuganti G, Ho navar S, Ahmed N, Hasnain S, Kanna biran C. Mutational analysis of the RB1gene in Indian patients with retinoblastoma. Ophthalmic Genet. 2002;23:1218.
6. Kiran VS, Kannabiran C, Chakravarthi K, Vemuganti GK, Honavar SG. Mu tational screening of the RB1 gene in Indian patients with retinoblastoma re veals eight novel and several recurrent mutations. Hum Mutat. 2003;22:339.
7. Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT, Naduvilath TJ. Factors predictive of recurrence of retinal tumors, vitreous seeds, and sub retinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460-4.
8. Shields CL, Honavar SG, Meadows AT, Shields JA, Demirci H, Singh A, Friedman DL, Naduvilath TJ. Chemoreduction plus focal therapy for retino blastoma: factors predictive of need for treatment with external beam radio therapy or enucleation. Am J Ophthalmol. 2002;133:657-64.
9. Shields CL, Honavar SG, Meadows AT, Shields JA, Demirci H, Naduvilath TJ. Chemoreduction for unilateral retinoblastoma. Arch Ophthalmol. 2002;120:1653-8.
10. Murthy R, Honavar SG, Naik MN, Reddy VA. Retinoblastoma. In: Dutta LC, ed. Modern Ophthalmology . New Delhi, India, Jaypee Brothers; 2004:849859.
11. Honavar SG, Singh AD. Manage ment of advanced retinoblastoma. Ophthalmol Clin North Am. 2005;18:6573.
12. Vemuganti G, Honavar SG, John R. Clinicopathological profile of retino blastoma in Asian Indians. Invest Ophthalmol Vis Sci 2000; 41(S):790.
13. Honavar SG, Singh AD, Shields CL, Meadows A, Shields JA. Does adjuvant chemotherapy prevent metastasis in high-risk retinoblastoma? Investigative Ophthalmology and Visual Science, 2000, 41(S):790
14. Honavar SG, Rajeev B. Needle Tract Tumor Cell Seeding Following Fine Nee dle Aspiration Biopsy for Retinoblasto ma. Investigative Ophthalmology and Visual Science 1998; 39: S 658.
15. Honavar SG, Shields CL, Shields JA, Demirci H, Naduvilath TJ. Intraocular surgery after treatment of retinoblast -oma. Arch Ophthalmol. 2001;119:161321.
16. Shields CL, Honavar S, Shields JA, Demirci H, Meadows AT. Vitrectomy in eyes with unsuspected retinoblastoma. Ophthalmology. 2000;107:2250-5.
17. Honavar SG, Reddy VAP, Murthy R, Naik M, Vemuganti GK. Management of orbital retinoblastoma. XI Interna tional Congress of Ocular Oncology. Hyderabad, India, 2004. pp. 51.
18. Stallard HB: The conservative treatment of retinoblastoma. Trans Am Op ththalmol Soc UK 1962; 82:473-534.
19. Murphree AL, Benedict WF: Retinoblastoma: Clues to human oncogenesis. Science 1984: 223:1028-1033.
20. Knudson AG: Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci, USA 1971:68: 820-823.
21. Abramson DH, Frank CM, Susman M, Whalen MP, Dunkel IJ, Boyd NW 3rd. Presenting signs of retinoblastoma. J Pediatr. 1998; 132:505-8.
22. Ellsworth RM. The practical man agement of retinoblastoma. Trans Am Ophthalmol Soc 1969: 67: 462-534.
23. Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18:41-53.
24. Shields CL, Shields JA. Basic under standing of current classification and management of retinoblastoma. Curr Opin Ophthalmol.2006;17:228-34.
25. Chantada G, Doz F, Antoneli CB, et al A proposal for an international retinoblastoma staging system Pediatr Blood Cancer 2006:47;801-805
26. Shields CL, Santos MC, Diniz W et al. Thermotherapy for retinoblastoma. Arch Ophthalmol 1999; 117: 885-893.
27. Shields CL, Shields JA, Cater J et al. Plaque radiotherapy for retinoblas toma, long term tumor control and treatment complications in 208 tumors. Ophthalmology 2001;108: 2116-2121.
28. Ellsworth RM. Retinoblastoma. Modern problems in ophthalmology. 1977; 96:1826-1830.
29. Hungerford JL, Toma NMG, Plow man PN, Kingston JE. External beam ra diotherapy for retinoblastoma: I, wholeeye technique. Br J Ophthalmol. 1995; 79: 112-117.
30. Abramson DH, Frank CM. Second nonocular tumors in survivors of bilat eral retinoblastoma; a possible age effect on radiation-related risk. Ophthal mology. 1998; 105: 573-580.
31. Shields JA, Shields CL, Sivalingam V. Decreasing frequency of enucleation in patients with retinoblastoma. Am J Ophthalmol 1989; 108:185-8.
32. Ferris FL, 3rd, Chew EY. A new era for the treatment of retinoblastoma. Arch Ophthalmol 1996; 114:1412.
33. Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Archives of Ophthalmology 1996; 114:1339-43.
34. Gallie BL, Budning A, DeBoer G, Thiessen JJ, Koren G, Verjee Z, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma with out radiotherapy. Arch Ophthalmol 1996; 114:1321-8.
35. Murphree AL, Villablanca JG, Deegan WF, 3rd, Sato JK, Malogolowkin M, Fisher A, et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Oph thalmol 1996; 114:1348-56.
36. Shields CL, De Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol 1996; 114:1330-8.
37. Honavar SG, Shome D, Reddy VAP. Periocular carboplatin in the manage ment of advanced intraocular retin oblastoma. Proceedings of the XII Intrernational Congress of Ocular On cology, Vancouver, Canada, 2005.
38. Abramson DH, Niksarli K, Ellsworth RM, Servodidio CA. Changing trends in the management of retinoblastoma: 1951-1965 vs 1966-1980. J Pediatr Ophthalmol Strabismus 1994; 31:32-7.
39. Singh AD, Shields CL, Shields JA. Prognostic factors in retinoblastoma. J Pediatr Ophthalmol Strab 2000; 37:13441.
40. Ajaiyeoba IA, Akang EE, Campbell OB, Olurin IO, Aghadiuno PU. Retino blastomas in Ibadan: treatment and prognosis. West African Journal of Medicine 1993; 12:223-7.
41. Kingston JE, Hungerford JL, Plow man PN. Chemotherapy in metastatic retinoblastoma. Ophthalmic Paediatr Genet 1987; 8:69-72.
42. White L. Chemotherapy for retino blastoma: where do we go from here? Ophthalmic Pediatr and Genet 1991; 12:115-30.
43. Kopelman JE, McLean IW, Rosenberg SH. Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enucleation. Ophthalmology 1987; 94:371-7.
44. Magramm I, Abramson DH, Ellsworth RM. Optic nerve involvement in retinoblastoma. Ophthalmology 1989; 96:217-22.
45. Messmer EP, Heinrich T, Hopping W, de Sutter E, Havers W, Sauerwein W. Risk factors for metastases in patients with retinoblastoma. Ophthalmology 1991; 98:136-41.
46. Shields CL, Shields JA, Baez KA, et a. Choroidal invasion of retinoblastoma: Metastatic potential and clinical risk factors. Br J Ophthalmol 1993; 77:5448.
47. Shields CL, Shields JA, Baez K, Cater JR, De Potter P. Optic nerve invasion of retinoblastoma. Metastatic potential and clinical risk factors. Cancer 1994; 73:692-8.
48. Chantada GL, de Silva MTG, Fand ino A, et a. Retinoblastoma with low risk for extraocular relapse. Ophthalmic Genet 1999; 20:133-40.
49. Khelfaoui F, Validire P, Auperin A, Quintana E, Michon J, Pacquement H, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single insti tution. Cancer 1996; 77:1206-13.
50. Honavar SG, Singh AD, Shields CL, Demirci H, Smith AF, Shields JA. Postenucleation prophylactic chemotherapy in high- risk retinoblastoma. Arch Oph thalmol 2002; 120:923-31.