I. Introduction :
Anterior segment optical coherence tomography (ASOCT) is a sophisticated non-contact imaging device that produces high-resolution images for detailed assessment of the structures of the ocular surface and anterior chamber in various disease conditions. Starting from the earlier generation time-domain devices first described in 1991 by Huang et al [1] to the current Fourier-domain OCT devices, the technological advancement in this device has improved the diagnostic ability tremendously. This imaging modality has evolved into a highly precise tool for clinical diagnosis and management as well as in research and understanding disease pathology. In this review, we describe the evolution of ASOCT, its technology in brief and focus on highlighting the various clinical applications with examples.
While Izzat et al first introduced the use of AS-OCT for imaging the anterior segment of the eye [2], it is well known that the OCT initially evolved as a tool for retinal imaging. The light source for this device was from a near-infrared 800nm superluminescent diode (SLD). [3] However, when the same device was attempted to be used for anterior segment imaging, the imaging ability was limited by the short wavelength, poor penetration through opaque layers, and slow scanning speed (400 axial scans per second).[4] The incorporation of a higher wavelength (1310nm) emitting SLD improved the penetration and image acquisition speed.[2] Imaging of the ciliary body is still limited as the light waves penetrated higher wavelengths (1310nm) poorly through the posterior pigment layer of the iris.[2,4]
II. Principle of OCT [5]
- The device works on the principal of Michelson interferometer.
- A reference beam of infrared rays is used to measure the time delay and intensity of the infrared rays reflected from the various tissue layers of the eye. The various tissues are known to have variable reflectivity, depending upon the depth and structure.
- These reflected rays then collectively form an interference pattern. Multiple such interference patterns create a cross-sectional image of the tissue of interest.
- To obtain an OCT image, multiple such scans are performed at discrete locations to create multiple axial scans (A-scan), and these multiple axial scans are combined to form a complex two-dimensional image (B-scan).
III. Types of OCT [3-6]
At present, there are two main types of AS-OCT
- Time domain (TD-OCT) - Visante OCT (Carl Zeiss Meditec, Inc., Dublin, CA, USA) and slit-lamp OCT (Heidelberg Engineering GmbH, Heidelberg, Germany)
- Spectral domain/ Fourier domain (SD-OCT, FD-OCT) - Spectralis (Heidelberg Engineering GmbH, Heidelberg, Germany), RTVue (Optovue, Inc., CA, USA), and Cirrus OCT (Carl Zeiss Meditec, Oberkochen, Germany)
Time domain (TD OCT) |
Fourier domain (TD OCT) |
|
Multiple cross-sectional images (A-scan) are generated sequentially, one pixel at a time of 1.6 seconds |
A single A scan is generated based on Fourier transformation of spectrometer analysis |
|
Long-wavelength of light used - 1310nm
|
Short wavelength of light used - 830nm
|
|
Moving reference mirror
|
Stationery reference mirror
|
Newer OCTs:
Swept Source OCT (SS-OCT) [7]
CASIA SS-1000 OCT (Tomey, Nagoya, Japan) is a Fourier-domain, SS-OCT predominantly used for imaging the anterior segment. This device uses rapidly tunable laser as the emitting source of light, thus producing high-resolution images of 10 ?m (axial) and 30 ?m (transverse) and high-speed scanning of 30,000 A-scans per second. With this speed, the entire 360 degrees of the anterior chamber angle can be visualized in 2.4 seconds.
Ultrahigh-resolution OCT (UHR-OCT) [8-10]
UHR-OCT uses Ti : Al2O3 (sapphire) laser with a broad bandwidth of 800nm, giving a higher resolution of 1-4 microns, and a speed of 140,000 A-scans/second. These features offer, easy in-vivo imaging of the intra-corneal and intra-retinal structures, which was earlier possible only by histopathological examination of these issues, hence it is also called ‘optical biopsy’. Individual layers of the cornea and conjunctiva can be visualized including the tear film and the meniscus. This device offers the highest in-vivo resolution to date amongst all the other OCT’s used for ophthalmic imaging. It is extremely helpful in early diagnosis and management and differentiating various conditions which are not detected so early during the course of any disease, by other conventional OCT. It is also useful for tracking the progression and monitoring the therapeutic efficacy for various treatments in different ocular diseases like ocular surface squamous neoplasia and conjunctival melanoma.
Intra-operative OCT (iOCT) [11]
Handheld (Bioptigen, Inc., Research Triangle Park, NC) and microscope mounted OCT has been used in the pediatric age group and is easy to use even in the supine position. Images can be acquired only after the surgical procedure is paused. This may add to the surgical time and these images do not reflect actual tissue-instrument interaction. Whereas, an iOCT provides a real-time display of tissue-instrument interaction and do not interrupt the surgical procedure. This device helps to ascertain the depth of surgical plane, residual ocular lesions, and residual corneal thickness during lamellar corneal surgeries, without interrupting the surgery. The only limitation of this device is the back shadowing produced by the metallic instruments. iOCT is also useful in cataract surgery to assess the corneal wound integrity and IOL position intra-operatively.
The most common commercially available real-time AS-OCT are LenSx (Alcon LenSx Lasers Inc., Aliso Viejo, CA, USA), Catalys (Optimedica, Sunnyvale, CA USA), and VICTUS (Technolas Perfect Vision GmbH, Munich, Germany).
IV. Clinical applications of AS-OCT:
A. Dry eye evaluation [12]:
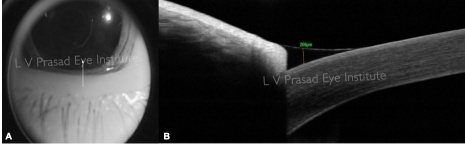
Tear Volume measurement
The tear meniscus is a wedge-shaped area measured between the posterior margin of the lower lid and the bulbar conjunctiva. The AS-OCT is a non-invasive technique to evaluate this. The parameters calculated are tear meniscus height (TMH) (Figure 1), tear meniscus depth (TMD) and tear meniscus area (TMA).[13] These parameters may help in differentiating a healthy ocular surface from that of a patient with dry eyes. These parameters may also be affected in patients with ectropion, lower lid mass, lid defects. This non-invasive technique offers some advantage over other conventional methods of assessment of dry eyes such as Schirmer’s test, which may have a few drawbacks- (1) Invasive and may stimulate reflex tearing (2) inter-observer variability. Quantification of the TMH can be done on AS-OCT. TMH has been found to increase significantly after the instillation of artificial tears and punctal occlusion, and decrease following punctoplasty and dacryocystorhinostomy in patients with epiphora.[13-15]
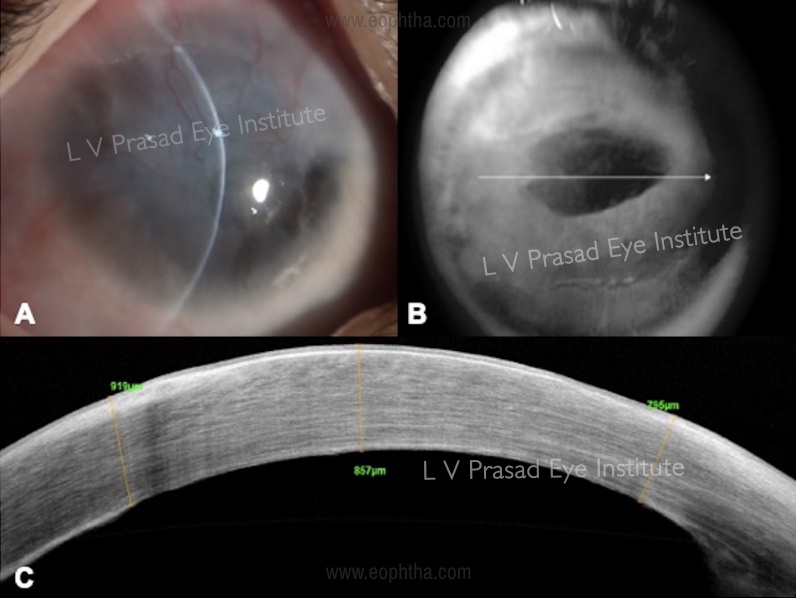
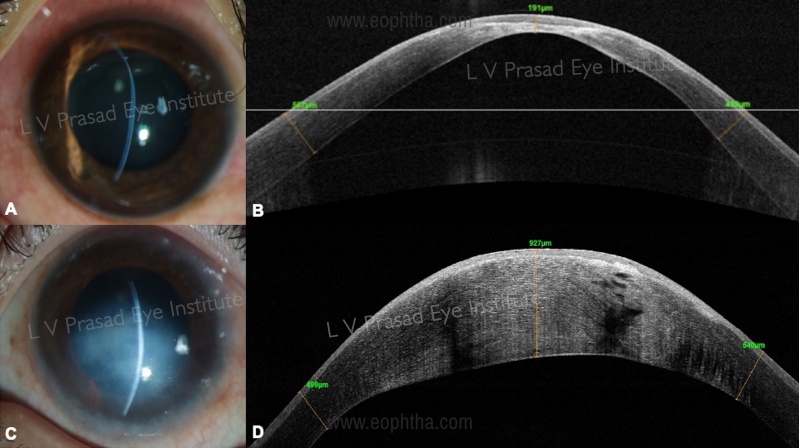
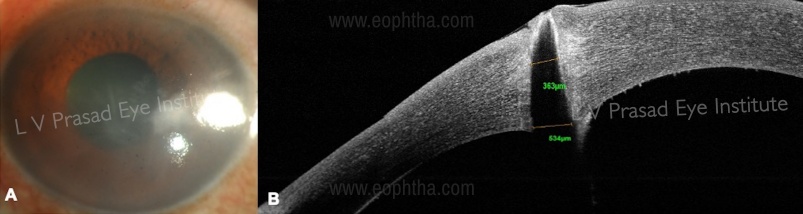
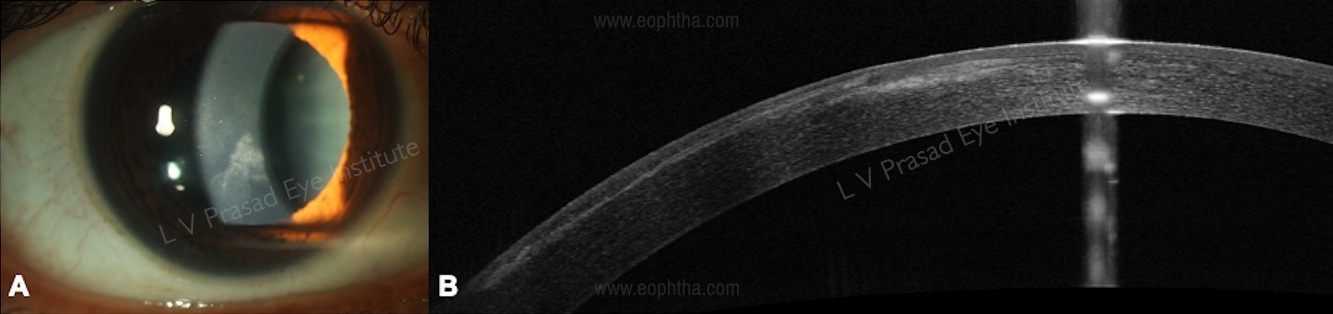
B. Assessment of corneal thickness and corneal scarring in patients with limbal stem cell deficiency (LSCD). [16]
Cases with total LSCD (Figure 2A) may present following chemical injury, chronic VKC, and others. In cases that have dense stromal opacification and extensive fibrovascular pannus, it is difficult to assess the condition of the underlying stroma. An AS-OCT can provide additional information such as:
- The extent of underlying stromal scarring can be estimated from the infrared image (Figure 2B). These cases need additional surgery such as full-thickness penetrating keratoplasty or anterior lamellar keratoplasty in addition to simple limbal epithelial transplant (SLET) to address the scarring, either as a single surgery or a staged procedure later.
- The extent of corneal thickness can be assessed from the linear scan (Figure 2C). Areas that are thin need careful dissection of pannus over them, as there is a risk of perforation in these areas
C. Conjunctival lesions [17,18]
HR-ASOCT imaging has been described in differentiating the nature of the conjunctival tissue growing over the cornea. While OCT is not required for the diagnosis, it can add diagnostic value in cases of doubt.
- Pterygium: In cases of primary pterygium there is a wedge-shaped growth of conjunctival tissue, seen as a hyperreflective layer, beneath the corneal epithelium separating it from the Bowman’s membrane (Figure 3A, B). In cases of pseudo-pterygium, the conjunctival tissue grows over the corneal epithelium and is not integrated in the corneal tissue.
- Pinguecula- the conjunctival hypertrophic epithelium abruptly ends at the limbus and does not grow over the cornea (Figure 3C, D).

D. Ocular surface tumors:
AS-OCT is a non-invasive, non-contact technique for imaging ocular surface tumors. Various quantitative parameters such as overall image quality of the lesions, posterior tumor shadowing, and tumor margins are better appreciated on UBM images as compared to AS-OCT images.[19] However, considering it a non-invasive and easier method of assessment, it is a useful technique to supplement the findings of non-pigmented anterior surface tumors. Whereas, ultrasound biomicroscopy (UBM) has shown better imaging features for large, heavily pigmented, and ciliary body tumors.[19]
Ocular surface squamous Neoplasia (OSSN) [20]
OSSN is a common epithelial surface tumor. The invaluable role of AS-OCT in the diagnosis and management of OSSN has been established in various studies. The classical features of OSSN described are (Figure 4 A, B)
- hyperreflective thickened epithelium
- abrupt transition from normal to abnormal epithelium
- well-defined plane between the lesion and underlying tissue.
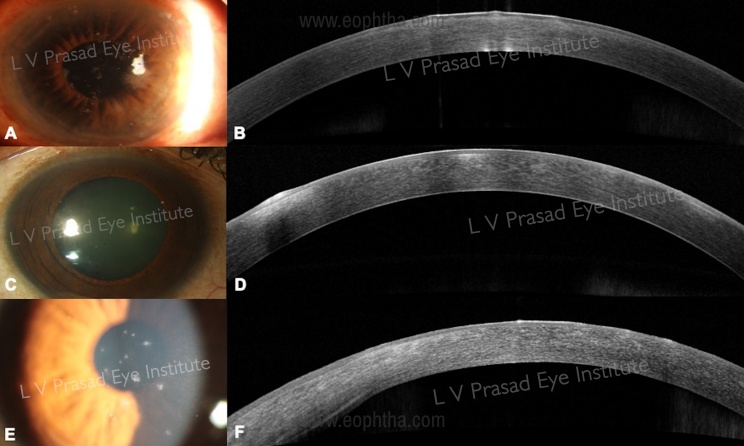
AS-OCT is also useful in assessing early recurrence of the disease after medical or surgical excision of the lesion, when it may appear to have resolved clinically. Also, AS-OCT helps to differentiate other lesions, such as corneal OSSN (Figure 4 C, D), pinguecula, and actinic keratosis (Figure 4 E, F) that maybe occasionally be difficult to clinically differentiate from OSSN.

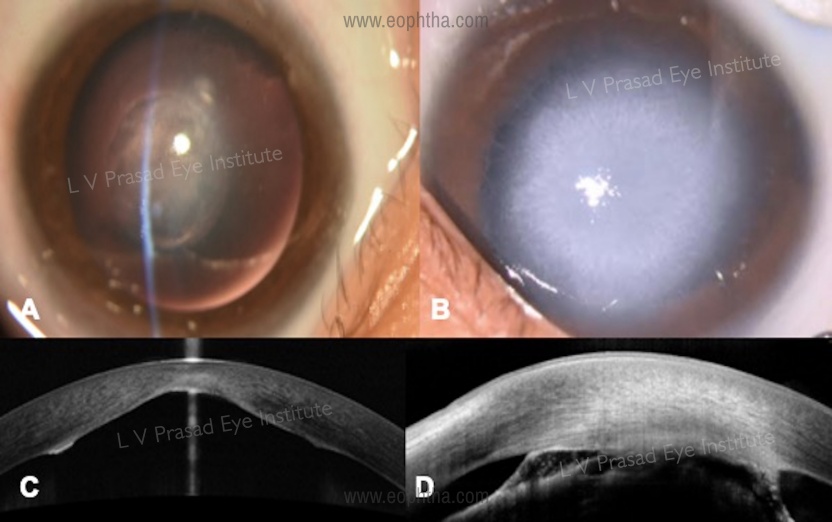
E. Congenital corneal opacities [21]
Congenital corneal opacity is a common feature of various conditions associated with anterior-segment dysgeneses like Peters’ anomaly, sclerocornea, corneal plana, and others. It is important to differentiate each clinical entity from the other as the prognosis is different in each case. Timely surgical intervention for visual rehabilitation in children is important to prevent permanent visual loss. AS-OCT helps us to understand the involvement of the iris and the crystalline lens and determine the presence of irido-lenticular and iridocorneal adhesions (Figure 5). Surgical intervention in the form of optical iridectomy or penetrating keratoplasty is decided based on the other associated anterior segment dysgenesis.
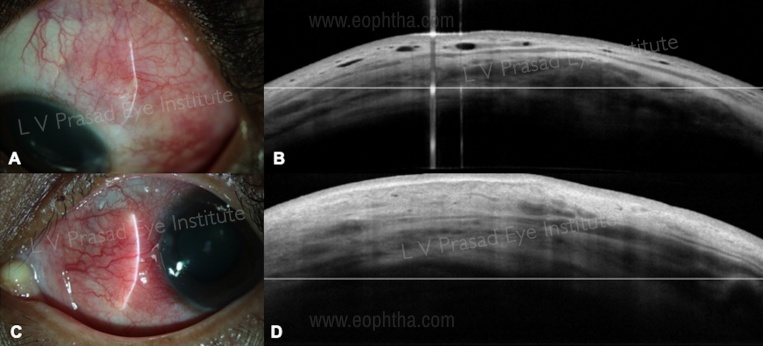
F. Sclera: episcleritis and scleritis
AS-OCT is an easy non-contact imaging modality that helps to identify the changes associated with scleral inflammation. In episcleritis, there is diffuse thickening limited to the conjunctiva and episcleral tissue. The hypo-reflective areas represent edema and dilated diffuse vessels are seen in the superficial episcleral plexus (Figure 6 A, B). These vessels do not extend in the scleral or deeper layers.[22] In scleritis, the thickening and increased reflectivity extend from the episcleral and scleral tissue, with dilatation of vessels both in the superficial and deep vascular plexus (Figure 6 C, D). These vessels extend into the episcleral tissue.[22]. With therapy, the scleral edema is noted to decrease and return to normal reflectivity over time.
G. Corneal indications:
Probably the largest contribution of this device is in corneal imaging. Invaluable both in clear and hazy corneas for various conditions: assessment of thickness, location of the pathology in the cornea, preoperative planning for various surgeries, pre and post-operative diagnosis
1. Keratoconus and other ectatic corneal diseases:
a.Diagnosis of keratoconus: AS-OCT has been known to serve as an adjunct tool in the diagnosis and management of corneal ectasias. The features suggestive of keratoconus on (Figure 7 A, B) AS-OCT [23] include:
- Focal, eccentric corneal thinning
- Asymmetric thinning between superior and inferior quadrants
- Corneal epithelial thickness maps have been useful in the early diagnosis of keratoconus. [24] Apical epithelial thinning in the typical inferotemporal cone has a corresponding epithelial thickening in the superonasal area. In the non-ectatic cornea, the epithelium is thinner superiorly and thicker inferiorly. A decrease in the epithelial thickness over a period of time is also noted in overtime when the patients are followed up serially. Changes in these parameters over time may also help in the diagnosis of progression.
However, there are limitations of OCT as a stand-alone diagnostic modality [23,24]. These indices cannot serve as independent parameters for the diagnosis of keratoconus as the epithelial thickness and reflectivity are often affected by eye rubbing. In addition, the Bowman’s layer is often fragmented, thus resulting in the poor demarcation between the epithelium and the Bowman’s layer on the As-OCT image
b. Role of AS-OCT in collagen cross-linking (CXL)
- CXL is a laser ablative procedure that is known to stop or retard the progression in keratoconus. Post CXL, a ‘demarcation line’ is seen as a hyperreflective layer between the anteriorly placed treated corneal stroma and the posterior untreated corneal stroma.[25] The depth of this ‘demarcation line’ line can be assessed on AS-OCT.[25,26] This line has been found to be present at variable depths based on the different CXL protocols.[27]
- The anterior treated stroma post CXL appears to be hyper-reflective and presumably due to increasing in the extra-cellular matrix production and hyperplasia of the keratocytes.[25-27]
- A secondary demarcation line may also be sometimes seen on AS-OCT.[28] This line is seen as a faint hyper-reflective line at variable depth, due to differences in the depth of laser treatment as a result of variable corneal thickness. [28]
c.Cornea hydrops [29] (Figure 7 C, D)
Hydrops is a complication of keratoconus, where a break in Descemet’s membrane is followed by leakage of aqueous humor into the corneal stroma. AS-OCT helps us to assess the location, size, and morphology of the break, and its evolution with time. The success of intervention for acute hydrops such as descemetopexy can also be studied post-operatively.
d.Intra-corneal ring segments (ICRS)
ICRS is placed in the mid-peripheral corneal stroma, to induce flattening of the ectatic corneal surface. However, poor placement can result in a number of complications.[30] Anterior placement can lead to extrusion of the ring segments, epithelial breakdown with secondary infection and posterior placement may lead to extrusion of the segments in the anterior chamber following perforation. The adequate depth at which the ICRS is required to be placed, may not be assessed accurately on the slit lamp, thus AS-OCT can be extremely useful in these cases.[31] Also, monitoring the migration and location of these segments over time can be done by serial AS-OCT images.[30,31]
e.Corneal transplant
Deep anterior lamellar keratoplasty (DALK) and penetrating keratoplasty (PK) are the two most common corneal transplantation procedures performed for the treatment of keratoconus. AS-OCT is useful while planning, performing, and postoperative follow-up of these cases.[32,33]
Pre-operative: 1. Assessing corneal thickness. 2.Presence of corneal scarring (as can be noted after healed hydrops). 3.Depth of corneal scar (this is important as the formation of big-bubble in DALK will be prevented by full-thickness corneal scar)[33]
Intra-operative: 1.While performing manual dissection in DALK, AS-OCT is useful to ensure minimal residual stroma is left behind. 2.In the case of DM perforation, whether there is any ingress of fluid into the interface, is likely to affect the postoperative outcome.[34]
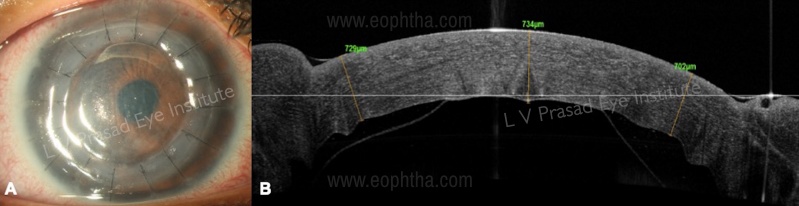
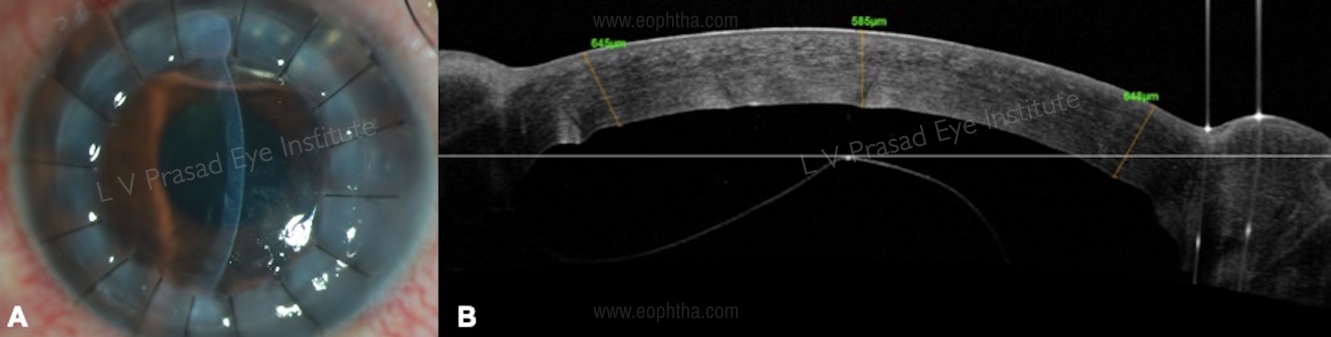
Post-operative : 1. To look for graft-host junction apposition, corneal curvature. 2.Complications following DALK- Double anterior chamber due to DM perforation intra-operatively, DM detachment and to intervene immediately for better prognosis.[34] (Figure 8 A, B)
2. Corneal infections:
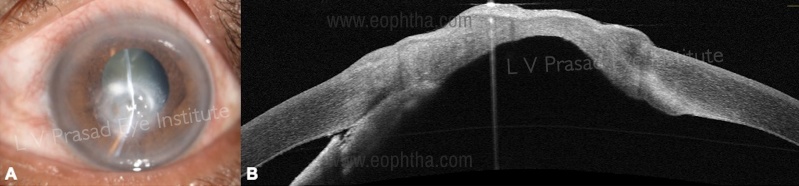
a.Corneal stromal melt - early prediction of impending corneal perforation and corneal descemetocele, which may be sometimes difficult to assess on slit-lamp examination as the patient may have severe photophobia.[35] (Figure 9 A, B)
b.Performing therapeutic DALK for microbial keratitis, in cases that do not respond to medical therapy. AS-OCT is useful to assess the depth of corneal infiltrate for better surgical outcomes and prevention of recurrences.[36]
c.AS-OCT has been useful in cases of endothelitis (HSV, CMV) to assess the corneal thickness and the nature of keratic precipitates.
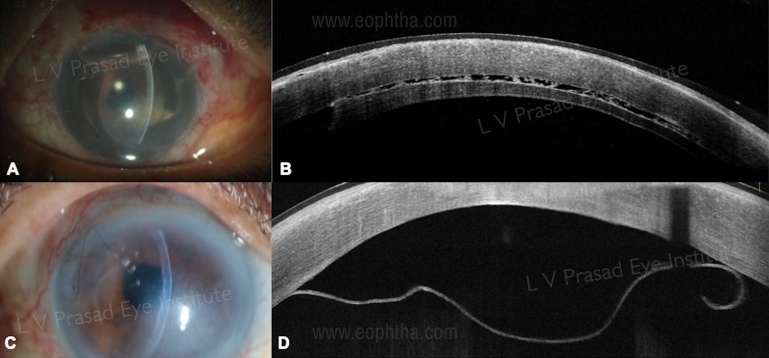
d.To study morphology and the level of corneal lesions in superficial punctate keratitis in microsporidial keratitis, adenoviral keratitis, and Thygesons’ superficial punctate keratitis. (Figure 10)
e. Nature and depth of a retained/residual foreign body within the cornea with surrounding infection can be visualized more easily on AS-OCT as compared to slit-lamp biomicroscopy. (Figure 11)
3. Utility of AS-OCT in corneal transplantation:
DALK :
i. Pre-operatively [37] for assessment of central and peripheral corneal thickness: Achievement of big-bubble formation during DALK surgery enables a relatively shorter and easier surgery and is associated with better post-operative outcomes Adequate trephination of depth and central insertion of air cannula at the correct depth without damaging the DM is paramount for this technique. Pneumatic dissection is also dependent on the density and level of stromal scarring, which can be determined by the AS-OCT, pre-operatively.
ii. Intra-operative: Intra-operative AS-OCT ensures that the air cannula has reached adequate depth, thus increasing the chances of big-bubble formation.[38] Real-time intra-operative AS-OCT helps in visualizing every step of the surgery performed, thus ensuring adequate depth of corneal trephination and air-cannula insertion. Also, micro-perforations can be easily picked up intra-operatively, thus avoiding dissection of the corneal stroma in these areas.
iii. Post-operative complications [37-39]: Quantitative assessment of these complications, and follow up post-operatively is easily done using AS-OCT
-Pseudo-anterior chamber formation (Figure 12): This may occur due to intra-operative perforation of the DM, which results in seepage of aqueous into a potential space created between the graft and the recipient DM. This may be missed on slit-lamp examination, however, it is advisable to rule this out using AS-OCT in all cases which have had an intra-operative DM perforation.
-Triple chamber formation can occur when there is perforation in Dua’s layer and DM together resulting in the accumulation of aqueous humor in potential spaces created between these layers.
-Interface keratitis
Endothelial keratoplasty (EK)
i. Pre-operative: the decision between PK versus Descemet Stripping Automated endothelial keratoplasty (DSAEK): the presence of a dense pannus within the sub-epithelium layer can prevent view of the anterior chamber, leading the clinician to plan a PK rather than EK. Detection of stromal scarring, epithelial hyperplasia (in chronic cases of endothelial decompensation), sub-epithelial fibrosis and scarring is known to affect the outcome of any endothelial keratoplasty.[40-44] These features are better appreciated on AS-OCT.
ii. Intra-operative: real-time AS-OCT images are helpful in cases with dense stromal edema, thus helping in better visualization and maneuvering of the graft. Also, the presence of inter-face fluid can be assessed intra-operatively, which is a common cause for failure of graft attachment.[41] Venting incisions can be placed more accurately using Intra-operative AS-OCT and egress of the interface fluid can be better ascertained.[41]
iii. Post-operative assessment [42-46]
-Graft detachment (Figure 13) – AS-OCT is useful in identifying localized or total graft detachment, especially in the presence of corneal edema where it is difficult to visualize anterior chamber details.
-Identification of risk factors for graft failure: inverse graft implantation in DSAEK or Descemet membrane endothelial keratoplasty (DMEK), presence and location of residual host cornea DM, eccentric trephination, etc.
-Interface fluid: presence and location of the fluid and absorption over time (Figure 13 A, B)
-Serial monitoring of donor graft and recipient cornea thickness- suggestive of endothelial cell function and prognosis of the surgery.
-Epithelial ingrowth after DSAEK
-Interface keratitis
4. Utility of AS-OCT in refractive surgery
a. Phakic intraocular lens (IOL): the AS-OCT has several uses in patients undergoing phakic IOL implantation [47]
i. Pre-operative evaluation of anterior segment structures especially the peripheral angles
ii. Post-operatively, AS-OCT is useful to assess the position of the phakic IOL, measurement of the vault of the lens and ruling out flipped lens implantation or other complications such as contact of the implant with the crystalline lens that can lead to cataract [47] (Figure 14)
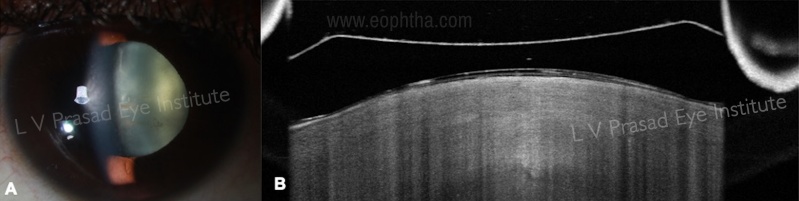
iii. Iris claw phakic IOL: pre-operative criteria is anterior chamber depth (ACD) of 2.8mm and CLR of < 300microns>
b. AS-OCT in kerato-refractive surgery (Laser in situ keratomileuses, LASIK)
i. To understand the shape of a LASIK flap. Planar-shaped flaps provide better biomechanical stability as compared to meniscus-shaped flaps [49]
ii. Measurement of thickness of the flap and residual stromal bed thickness, which is important both as a pre-operative criterion and useful for planning in enhancement procedures. [49]
iii. Intra-operative hand-held OCT (Bioptigen Inc., Morrisville, NC, USA) is extremely useful to evaluate the flap thickness and stromal bed thickness before stromal hydration sets in.[50]
iv. Complications: AS-OCT is a useful tool in diagnosis, management, and post-treatment follow-up of flap-related complications like interface fluid syndrome (IFS), epithelial ingrowth (Figure 15), lamellar keratitis, infectious keratitis. [51]
5. AS-OCT in femtosecond laser-assisted surgery
a. Femtosecond laser-assisted cataract surgery (FLACS) Intra-operative OCT is useful in proper centration over the visual axis, accurate corneal incisions, capsulotomy, and lens fragmentation
b. Femtosecond laser-assisted lamellar keratoplasty (FALK): the sidecut and depth can be planned using the femto laser, and these can be accurately assessed by the OCT.
6.AS-OCT in corneal scars and corneal dystrophies [52,53]
a. Planning a surgical procedure: AS-OCT is used to ascertain the depth of deposits/ stromal haze, in cases of stromal dystrophy and corneal scars ( Figure 16). Depending on the depth, either a full-thickness penetrating keratoplasty or a lamellar keratoplasty can be performed
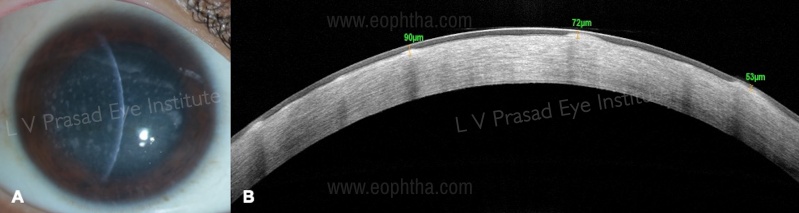
b. AS-OCT determines the size and location of corneal scars. Superficial lesions within 100 microns can be effectively treated with phototherapeutic keratectomy (PTK)
6. AS-OCT in cataract surgery :
a. Pre-operative: AS-OCT is useful in IOL power calculation, assessment of the angle structures, the crystalline lens and other risk factors for cataract surgery if present.[54] It has shown promising results in assessing IOL power calculation in eyes undergoing cataract surgery, post-refractive surgery.[55]
b. AS-OCT can be used to analyze the integrity, structure, and configuration of corneal wound incisions intra-operatively (iOCT)
c. Post-operative assessment :
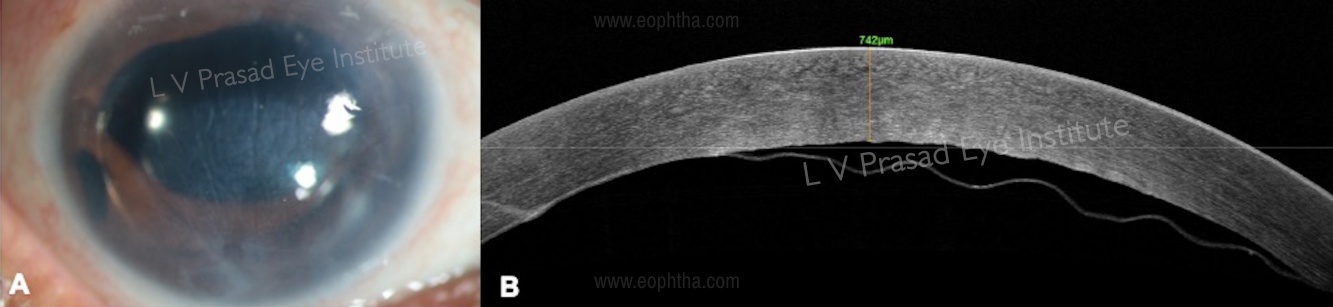
i. Descemet membrane detachment (DMD): DMD is suspected in cases with unexpected or prolonged corneal edema. Some DMDs may attach spontaneously, while some need surgical intervention. The location, configuration (planar/non-planar), and extent of DMD is better assessed on AS-OCT as compared to slit-lamp evaluation, especially in the setting of corneal edema.[56] (Figure 17) Also, these factors help in planning the surgical approach for these cases. The direction of intra-cameral injection of air or C3F8 is important to unroll the detached DM for a successful outcome. AS-OCT plays a pivotal role in timely intervention and post-operative follow-up of these cases, thus preventing permanent endothelial damage.
ii. IOL position and stability/ IOL tilting can be assessed to some extent by the AS-OCT, although the UBM is a better tool for this indication [57]
iii. Capsular block syndrome.[58]
7.Use of AS-OCT in glaucoma [59]
a. Assessment of anterior chamber angle and Laser peripheral iridotomy :
AS-OCT is a non-contact imaging modality to assess various parameters of the anterior chamber angle (ACA). These parameters are the angle opening distance (AOD 500 and 750????m), and trabecular-iris space area (TISA). Lesser inter-observer variability makes AS-OCT a reliable tool that is extremely useful to –
i. Screen narrow-angle eyes, more susceptible to develop angle-closure
ii. Identify structural abnormalities like plateau iris syndrome, pupillary block, and malignant glaucoma.
iii. To study the change in dynamics and ACA parameters post-laser iridotomy.
b. Assessment of filtering bleb after trabeculectomy :
i. AS-OCT is used to study the bleb morphology and other features which are associated with better control of IOP like bleb wall uniformity, low reflectivity of bleb wall, and presence of epi-scleral fluid.
ii. To ascertain which blebs require re-needling and following the procedure to study, the changes in the bleb morphology
iii. To confirm tube-erosion post glaucoma valve surgery, especially when there is thin overlying conjunctiva which is difficult to see on a slit-lamp evaluation. Thus AS-OCT helps to confirm a ‘true’ tube erosion.
V. Conclusion
AS-OCT is an extremely valuable non-contact, fast and reliable imaging-modality for diagnosis and management of various ocular conditions as described above. Operating the machine and interpretation the images has a short learning curve. With the advent of new technologies like ultrahigh resolution-OCT and iOCT, early diagnosis, prevention, and treatment of various conditions have been made possible.
References
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science.1991 Nov;254 (5035):1178–1181.
- ?J. A. Izatt, M. R. Hee, E. A. Swanson, “Micrometer-scale resolution imaging of the anterior eye in vivo with optical ?coherence tomography,”. Archives of Ophthalmology.1994; 112(12):1584–1589.
- M. Doors, T. T. J. M. Berendschot, J. de Brabander, C. A. B. Webers, and R. M. M. A. Nuijts, “Value of optical coherence tomography for anterior segment surgery,” Journal of Cataract and Refractive Surgery. 2010; 36(7):1213–1229.
- Leung CK, Chan WM, Ko CY, Chui SI, Woo J, Tsang MK, Tse RK. Visualization of anterior chamber angle dynamics using optical coherence tomography. Ophthalmology. 2005 Jun;112(6):980–984.
- ?J. L. B. Ramos, Y. Li, and D. Huang, “Clinical and research applications of anterior segment optical coherence tomography—a review,” Clinical and Experimental Ophthalmology.2009; 37(1): 81–89.
- Han SB, Liu YC, Noriega KM, Mehta JS. Applications of Anterior Segment Optical Coherence Tomography in Cornea and Ocular Surface Diseases. J Ophthalmol. 2016;2016:4971572.
- ?M. T. Leite, H. L. Rao, L. M. Zangwill, R. N. Weinreb, and F. A. Medeiros, “Comparison of the diagnostic accuracies of the spectralis, cirrus, and RTVue optical coherence tomography devices in glaucoma,” Ophthalmology. 2011; 118(7): 1334–1339.
- J. Wang, M. Abou Shousha, V. L. Perez et al., “Ultra-high resolution optical coherence tomography for imaging the anterior segment of the eye,” Ophthalmic Surgery, Lasers & Imaging. 2011; 42: S15–S27.
- ?Q. Chen, J. Wang, A. Tao, M. Shen, S. Jiao, and F. Lu, “Ultrahighresolution measurement by optical coherence tomography of dynamic tear film changes on contact lenses,” Investigative Ophthalmology and Visual Science. 2010; 51(4):1988–1993.
- Shousha MA, Karp CL, Canto AP, et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology. 2013; 120(5):883-891.
- Titiyal JS, Kaur M, Falera R. Intraoperative optical coherence tomography in anterior segment surgeries. Indian J Ophthalmol. 2017;65(2):116-121.
- Lim SH. Clinical applications of anterior segment optical coherence tomography. J Ophthalmol. 2015; 2015:605729.
- G. Czajkowski, B. J. Kaluzny, A. Laudencka, G. Malukiewicz, and J. J. Kaluzny, “Tear meniscus measurement by spectral optical coherence tomography,” Optometry and Vision Science. 212; 89(3): 336–342.
- S. E. Kim, S. J. Lee, S. Y. Lee, and J. S. Yoon, “Outcomes of 4-snip punctoplasty for severe punctal stenosis: measurement of tear meniscus height by optical coherence tomography,” American Journal of Ophthalmology. 2012; 153(4): 769.e2–773.e2, 2012.
- M. C. Bujak, S. Yiu, X. Zhang, Y. Li, and D. Huang, “Serial measurement of tear meniscus by FD-OCT after instillation of artificial tears in patients with dry eyes,” Ophthalmic Surgery Lasers and Imaging. 2011; 42(4): 308–313.
- Shanbhag SS, Patel CN, Goyal R, Donthineni PR, Singh V, Basu S. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J Ophthalmol. 2019;67(8):1265-1277.
- A. A. Nanji, F. E. Sayyad, A. Galor, S. Dubovy, and C. L. Karp, “High-resolution optical coherence tomography as an adjunctive tool in the diagnosis of corneal and conjunctival pathology,” Ocular Surface. 2015; 13(3): 226–235.
- W. Soliman and T. A. Mohamed, “Spectral domain anterior segment optical coherence tomography assessment of pterygium and pinguecula,” Acta Ophthalmologica. 2012;90(5): 461–465.
- C. Bianciotto, C. L. Shields, J. M. Guzman et al., “Assessment of anterior segment tumors with ultrasound biomicroscopy versus anterior segment optical coherence tomography in 200 cases,” Ophthalmology. 2011;118(7): 1297–1302.
- Thomas BJ, Galor A, Nanji AA, El Sayyad F, Wang J, Dubovy SR, et al. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 2014;12:46–58.
- Majander AS, Lindahl PM, Vasara LK, Krootila K. Anterior segment optical coherence tomography in congenital corneal opacities. Ophthalmology. 2012;119:2450–7.
- Axmann S, Ebneter A, Zinkernagel MS. Imaging of the Sclera in Patients with Scleritis and Episcleritis using Anterior Segment Optical Coherence Tomography. Ocul Immunol Inflamm. 2016;24(1):29-34.
- Yip H, Chan E. Optical coherence tomography imaging in keratoconus. Clin Exp Optom. 2019;102(3):218-223.
- Li Y, Tan O, Brass R et al. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology 2012; 119: 2425–2433.
- Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea 2006; 25: 1057–1059.
- Yam JC, Chan CW, Cheng AC. Corneal collagen cross- linking demarcation line depth assessed by Visante OCT after CXL for keratoconus and corneal ectasia. J Refract Surg 2012; 28: 475–481.
- Kymionis GD, Tsoulnaras KI, Grentzelos MA et al. Evaluation of corneal stromal demarcation line depth following standard and a modified-accelerated collagen cross-linking protocol. Am J Ophthalmol 2014; 158: 671–675.e1.
- Kymionis GD, Grentzelos MA, Plaka AD et al. Correlation of the corneal collagen cross-linking demarcation line using confocal microscopy and anterior segment optical coherence tomography in keratoconic patients. Am J Ophthalmol 2014; 157: 110–115.e1.
- Kucumen BR, Yenerel NM, Gorgun E et al. Anterior segment optical coherence tomography findings of acute hydrops in a patient with keratoconus. Ophthal- mic Surg Lasers Imaging 2010; 41: S114–S116.
- Coskunseven E, Kymionis GD, Tsiklis NS et al. Complications of intrastromal corneal ring segment implan- tation using a femtosecond laser for channel creation: a survey of 850 eyes with keratoconus. Acta Ophthal- mol 2011; 89: 54–57.
- Lai MM, Tang M, Andrade EM et al. Optical coherence tomography to assess intrastromal corneal ring segment depth in keratoconic eyes. J Cataract Refract Surg 2006; 32: 1860–1865.
- Arnalich-Montiel F, Alió Del Barrio JL, Alió JL. Corneal surgery in keratoconus: which type, which technique, which outcomes?. Eye Vis (Lond). 2016;3:2.
- Khurana RN, Li Y, Tang M et al. High-speed optical coherence tomography of corneal opacities. Ophthal- mology 2007; 114: 1278–1285.
- De Benito-Llopis L, Mehta JS, Angunawela RI et al. Intraoperative anterior segment optical coherence tomography: a novel assessment tool during deep anterior lamellar keratoplasty. Am J Ophthalmol 2014; 157: 334–341.e3.
- Soliman W, Fathalla AM, El-Sebaity DM, Al-Hussaini AK. Spectral domain anterior segment optical coherence tomography in microbial keratitis. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):549-553.
- Konstantopoulos A, Kuo J, Anderson D, Hossain P. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. Am J Ophthalmol. 2008;146(4):534-542.
- ? L. S. Lim, H. T. Aung, T. Aung, and D. T. H. Tan, “Corneal imaging with anterior segment optical coherence tomography for lamellar keratoplasty procedures,” American Journal of Ophthalmology. 2008;145(1):81–90.
- L. De Benito-Llopis, J. S. Mehta, R. I. Angunawela, M. Ang, and D. T. H. Tan, “Intraoperative anterior segment optical coherence tomography: a novel assessment tool during deep anterior lamellar keratoplasty,” American Journal of Ophthalmology. 2014;157(2): 334–341.
- V. Scorcia, M. Busin, A. Lucisano, J. Beltz, A. Carta, and G. Scorcia, “Anterior segment optical coherence tomographyguided big-bubble technique,” Ophthalmology.2013;120(3): 471–476.
- ?N. Sharma, S. Gupta, P. Maharana, P. Shanmugam, R. Nagpal, and R. B. Vajpayee, “Anterior segment optical coherence tomography-guided management algorithm for Descemet membrane detachment after intraocular surgery,” Cornea.2015;34(9):1170–1174.
- Miyakoshi A, Ozaki H, Otsuka M, Hayashi A. Efficacy of Intraoperative Anterior Segment Optical Coherence Tomography during Descemet's Stripping Automated Endothelial Keratoplasty. ISRN Ophthalmol. 2014;2014:562062
- K. Moutsouris, I. Dapena, L. Ham, C. Balachandran, S. Oellerich, and G. R. J. Melles, “Optical coherence tomography, scheimpflug imaging, and slit-lamp biomicroscopy in the early detection of graft detachment after Descemet membrane endothelial keratoplasty, Cornea.2011;30(12):1369–1375.
- E. Wylega?a and A. Nowinska, “Usefulness of anterior segment ´optical coherence tomography in Descemet membrane detachment,” European Journal of Ophthalmology. 2009;19(5): 723–728.
- G. D. Kymionis, T. Ide, K. Donaldson, and S. H. Yoo, “Diagnosis of donor graft partial dislocation behind the iris after DSAEK with anterior segment OCT,” Ophthalmic Surgery, Lasers an Imaging Retina.2010;42:E1–E2.
- R.-Y. Yeh, R. Quilendrino, F. U. Musa, V. S. Liarakos, I. Dapena, and G. R. J. Melles, “Predictive value of optical coherence tomography in graft attachment after descemet’s membrane endothelial keratoplasty,” Ophthalmology.2013;120(2): 240–245.
- C. Y. Shih, D. C. Ritterband, P.-M. Palmiero et al., “The use of postoperative slit-lamp optical coherence tomography to predict primary failure in descemet stripping automated endothelial keratoplasty,” American Journal of Ophthalmology.2009;147(5):796-800.
- Baïkoff G. Anterior segment OCT and phakic intraocular lenses: a perspective. J Cataract Refract Surg. 2006;32(11):1827-1835.
- Sinha R, Kumar C, Titiyal JS. Phakic intraocular lenses. Indian J Ophthalmol. 2009; 57: 165-9.
- Li Y., Netto M. V., Shekhar R., Krueger R. R., Huang D. A longitudinal study of LASIK flap and stromal thickness with high-speed optical coherence tomography. Ophthalmology. 2007;114(6):1124–1132
- Shetty R., Malhotra C., D'Souza S., Wadia K. WaveLight FS200 vs Hansatome LASIK: intraoperative determination of flap characteristics and predictability by hand-held bioptigen spectral domain ophthalmic imaging system. Journal of Refractive Surgery. 2012;28(supplement 11):S815–S820
- Han S. B., Woo S. J., Hyon J. Y. Delayed-onset interface fluid syndrome after laser in situ keratomileusis secondary to combined cataract and vitreoretinal surgery. Journal of Cataract and Refractive Surgery. 2012;38(3):548–550.
- ?Sridhar MS, Martin R. Anterior segment optical coherence tomography for evaluation of cornea and ocular surface. Indian J Ophthalmol 2018;66:367-72.
- ?Siebelmann S, Scholz P, Sonnenschein S, Bachmann B, Matthaei M, Cursiefen C, et al. Anterior segment optical coherence tomography for the diagnosis of corneal dystrophies according to the IC3D classification. Surv Ophthalmol 2017;9:S0039?6257(17)30164?9.
- Wong A. L., Leung C. K.-S., Weinreb R. N., et al. Quantitative assessment of lens opacities with anterior segment optical coherence tomography. British Journal of Ophthalmology. 2009;93(1):61–65
- Tang M., Wang L., Koch D. D., Li Y., Huang D. Intraocular lens power calculation after previous myopic laser vision correction based on corneal power measured by Fourier-domain optical coherence tomography. Journal of Cataract and Refractive Surgery. 2012;38(4):589–594
- Zhou SY, Wang CX, Cai XY, Liu YZ. Anterior segment OCT-based diagnosis and management of Descemet's membrane detachment. Ophthalmologica. 2012;227(4):215-222.
- Kumar D. A., Agarwal A., Prakash G., Jacob S., Saravanan Y., Agarwal A. Evaluation of intraocular lens tilt with anterior segment optical coherence tomography. American Journal of Ophthalmology. 2011;151(3):406–412.e2.
- Mastropasqua L., Toto L., De Nicola G., Nubile M., Carpineto P. OCT imaging of capsular block syndrome with crystalline cortical remnants in the capsular bag. Ophthalmic Surgery Lasers and Imaging. 2009;40(4):399–402.
- Li H, Jhanji V, Dorairaj S, Liu A, Lam DS, Leung CK. Anterior Segment Optical Coherence Tomography and its Clinical Applications in Glaucoma. J Curr Glaucoma Pract. 2012;6(2):68-74.

